
Aqua regia is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but it turns yellow, orange or red within seconds from the formation of nitrosyl chloride and nitrogen dioxide. It was so named by alchemists because it can dissolve noble metals like gold and platinum, though not all metals.

Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula HgO. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found.

Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula Pb3O4. A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one.
Cyanogen iodide or iodine cyanide (ICN) is a pseudohalogen composed of iodine and the cyanide group. It is a highly toxic inorganic compound. It occurs as white crystals that react slowly with water to form hydrogen cyanide.
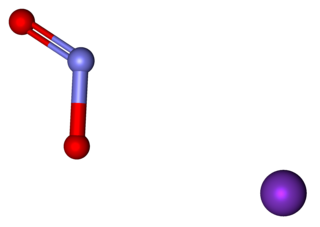
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula KNO2. It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water.

Lead(IV) oxide, commonly known as lead dioxide, is an inorganic compound with the chemical formula PbO2. It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms. It has several important applications in electrochemistry, in particular as the positive plate of lead acid batteries.
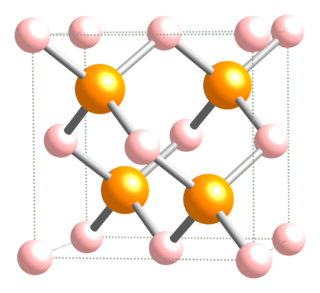
Aluminium arsenide is a semiconductor material with almost the same lattice constant as gallium arsenide and aluminium gallium arsenide and wider band gap than gallium arsenide. (AlAs) can form a superlattice with gallium arsenide (GaAs) which results in its semiconductor properties. Because GaAs and AlAs have almost the same lattice constant, the layers have very little induced strain, which allows them to be grown almost arbitrarily thick. This allows for extremely high performance high electron mobility, HEMT transistors, and other quantum well devices.
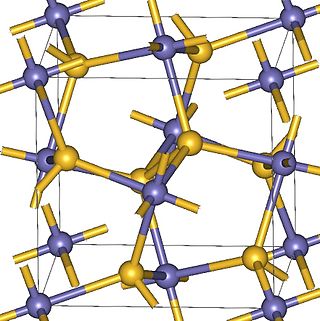
Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc peroxide has also been used as an oxidant in explosives and pyrotechnic mixtures. Its properties have been described as a transition between ionic and covalent peroxides. Zinc peroxide can be synthesized through the reaction of zinc chloride and hydrogen peroxide.
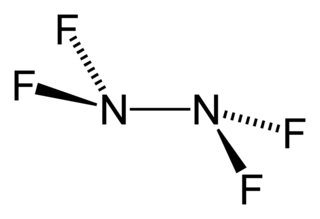
Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, nonflammable, reactive inorganic gas. It is a fluorinated analog of hydrazine.

Potassium nitrate is an oxidizer so storing it near fire hazards or reducing agents should be avoided to minimise risk in case of a fire.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove color from fabric or fiber or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
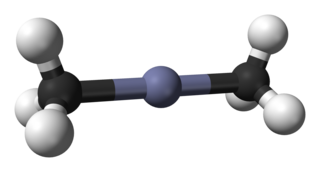
Dimethylzinc, also known as zinc methyl, DMZ, or DMZn, is a toxic organozinc compound with the chemical formula Zn(CH3)2. It belongs to the large series of similar compounds such as diethylzinc.
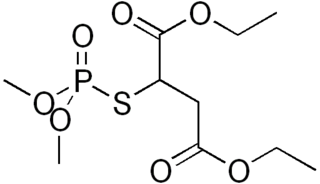
Malaoxon (Liromat, Malation oxon, Malthon oxon) is a chemical compound with the formula C10H19O7PS. More specifically, it is a phosphorothioate. It is a breakdown product of, and more toxic than, malathion.
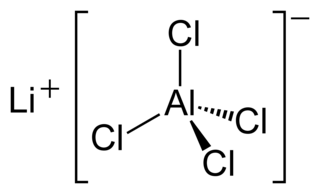
Lithium tetrachloroaluminate is an inorganic compound with the formula Li[AlCl4]. It consists of lithium cations Li+ and tetrahedral tetrachloroaluminate anions [AlCl4]−.
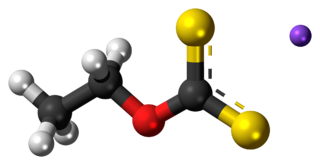
Sodium ethyl xanthate (SEX) is an organosulfur compound with the chemical formula CH3CH2OCS2Na. It is a pale yellow powder, which is usually obtained as the dihydrate. Sodium ethyl xanthate is used in the mining industry as a flotation agent. A closely related potassium ethyl xanthate (KEX) is obtained as the anhydrous salt.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.

Trifluoroacetyl chloride (also known as TFAC) is a toxic gaseous chemical compound with the chemical formula C2ClF3O. TFAC is the perfluorinated version of acetyl chloride. The compound is a gas, but it is usually shipped as a liquid under high pressure.

Dibutyltin dilaurate is an organotin compound with the formula (CH310CO2)2Sn(CH2CH2CH2CH3)2. It is a colorless viscous and oily liquid. It is used as a catalyst.

Stannoxane is a functional group in organotin chemistry with the connectivity SnIV−O−SnIV. Aside from the oxide group, usually 3 or 4 other substituents are attached to tin. In aqueous or aquatic environments, most organotin compounds contain this group.

Chlorine-releasing compounds, also known as chlorine base compounds, is jargon to describe certain chlorine-containing substances that are used as disinfectants and bleaches. They include the following chemicals: sodium hypochlorite, chloramine, halazone, and sodium dichloroisocyanurate. They are widely used to disinfect water and medical equipment, and surface areas as well as bleaching materials such as cloth. The presence of organic matter can make them less effective as disinfectants. They come as a liquid solution, or as a powder that is mixed with water before use.




















