
Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic in nanotechnology and materials science. When a quantum dot is illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when the band structure is no longer well-defined in QDs.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
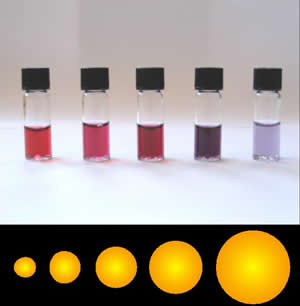
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red or blue-purple . Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.

A quantum dot solar cell (QDSC) is a solar cell design that uses quantum dots as the captivating photovoltaic material. It attempts to replace bulk materials such as silicon, copper indium gallium selenide (CIGS) or cadmium telluride (CdTe). Quantum dots have bandgaps that are adjustable across a wide range of energy levels by changing their size. In bulk materials, the bandgap is fixed by the choice of material(s). This property makes quantum dots attractive for multi-junction solar cells, where a variety of materials are used to improve efficiency by harvesting multiple portions of the solar spectrum.
Cell-penetrating peptides (CPPs) are short peptides that facilitate cellular intake and uptake of molecules ranging from nanosize particles to small chemical compounds to large fragments of DNA. The "cargo" is associated with the peptides either through chemical linkage via covalent bonds or through non-covalent interactions.
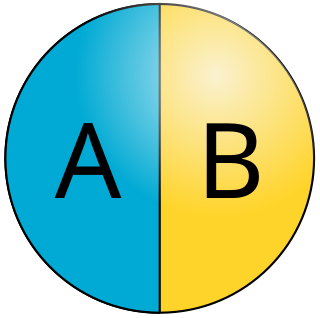
Janus particles are special types of nanoparticles or microparticles whose surfaces have two or more distinct physical properties. This unique surface of Janus particles allows two different types of chemistry to occur on the same particle. The simplest case of a Janus particle is achieved by dividing the particle into two distinct parts, each of them either made of a different material, or bearing different functional groups. For example, a Janus particle may have one half of its surface composed of hydrophilic groups and the other half hydrophobic groups, the particles might have two surfaces of different color, fluorescence, or magnetic properties. This gives these particles unique properties related to their asymmetric structure and/or functionalization.

Platinum nanoparticles are usually in the form of a suspension or colloid of nanoparticles of platinum in a fluid, usually water. A colloid is technically defined as a stable dispersion of particles in a fluid medium.

Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.

A nanoparticle–biomolecule conjugate is a nanoparticle with biomolecules attached to its surface. Nanoparticles are minuscule particles, typically measured in nanometers (nm), that are used in nanobiotechnology to explore the functions of biomolecules. Properties of the ultrafine particles are characterized by the components on their surfaces more so than larger structures, such as cells, due to large surface area-to-volume ratios. Large surface area-to-volume-ratios of nanoparticles optimize the potential for interactions with biomolecules.

Biomaterials are materials that are used in contact with biological systems. Biocompatibility and applicability of surface modification with current uses of metallic, polymeric and ceramic biomaterials allow alteration of properties to enhance performance in a biological environment while retaining bulk properties of the desired device.

Core–shell semiconducting nanocrystals (CSSNCs) are a class of materials which have properties intermediate between those of small, individual molecules and those of bulk, crystalline semiconductors. They are unique because of their easily modular properties, which are a result of their size. These nanocrystals are composed of a quantum dot semiconducting core material and a shell of a distinct semiconducting material. The core and the shell are typically composed of type II–VI, IV–VI, and III–V semiconductors, with configurations such as CdS/ZnS, CdSe/ZnS, CdSe/CdS, and InAs/CdSe Organically passivated quantum dots have low fluorescence quantum yield due to surface related trap states. CSSNCs address this problem because the shell increases quantum yield by passivating the surface trap states. In addition, the shell provides protection against environmental changes, photo-oxidative degradation, and provides another route for modularity. Precise control of the size, shape, and composition of both the core and the shell enable the emission wavelength to be tuned over a wider range of wavelengths than with either individual semiconductor. These materials have found applications in biological systems and optics.
The behavior of quantum dots (QDs) in solution and their interaction with other surfaces is of great importance to biological and industrial applications, such as optical displays, animal tagging, anti-counterfeiting dyes and paints, chemical sensing, and fluorescent tagging. However, unmodified quantum dots tend to be hydrophobic, which precludes their use in stable, water-based colloids. Furthermore, because the ratio of surface area to volume in a quantum dot is much higher than for larger particles, the thermodynamic free energy associated with dangling bonds on the surface is sufficient to impede the quantum confinement of excitons. Once solubilized by encapsulation in either a hydrophobic interior micelle or a hydrophilic exterior micelle, the QDs can be successfully introduced into an aqueous medium, in which they form an extended hydrogel network. In this form, quantum dots can be utilized in several applications that benefit from their unique properties, such as medical imaging and thermal destruction of malignant cancers.
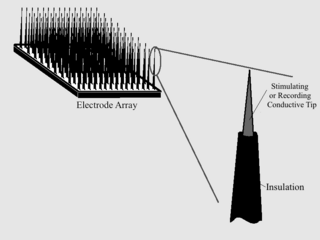
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.

Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
Quantum dots (QDs) are semiconductor nanoparticles with a size less than 10 nm. They exhibited size-dependent properties especially in the optical absorption and the photoluminescence (PL). Typically, the fluorescence emission peak of the QDs can be tuned by changing their diameters. So far, QDs were consisted of different group elements such as CdTe, CdSe, CdS in the II-VI category, InP or InAs in the III-V category, CuInS2 or AgInS2 in the I–III–VI2 category, and PbSe/PbS in the IV-VI category. These QDs are promising candidates as fluorescent labels in various biological applications such as bioimaging, biosensing and drug delivery.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.
Hedi Mattoussi is a Tunisian-American materials scientist and professor at Florida State University. His research considers colloidal inorganic nanocrystals for biological imaging and sensing. He is a Fellow of the American Physical Society, American Chemical Society and Materials Research Society.
A protein corona is a dynamic coating of biomolecules, usually proteins, around the surface of a nanoparticle that forms spontaneously in colloidal nanomaterials upon exposure to biological mediums. Protein coronas can form in many different patterns depending on their size, shape, composition, charge, and surface functional groups, and have properties that vary in different environmental factors like temperature, pH, shearing stress, immersed media composition, and exposing time. These coatings are also changeable according to the conditions of the biochemical and physiochemical surface interactions. Types of protein coronas are known to be divided into two categories: “hard” and “soft”. “Hard” coronas have higher-affinity proteins that are irreversibly bonded to the nanoparticle surface, while “soft” coronas have lower-affinity proteins on the nanoparticle surface that are reversibly bound. These reversibly-bound proteins allow for the biomolecules in “soft” protein coronas to be exchanged or detached over time for various applications. This process is governed by the intermolecular protein-nanoparticle and protein-protein interactions that exist within a solution. In "soft" protein coronas, it is common to observe an exchange of proteins at the surface; larger proteins with lower affinities will often aggregate to the surface of the nanoparticle first, and over time, smaller proteins with higher affinities will replace them, "hardening" the corona, known as the Vroman effect.
Silicon quantum dots are metal-free biologically compatible quantum dots with photoluminescence emission maxima that are tunable through the visible to near-infrared spectral regions. These quantum dots have unique properties arising from their indirect band gap, including long-lived luminescent excited-states and large Stokes shifts. A variety of disproportionation, pyrolysis, and solution protocols have been used to prepare silicon quantum dots, however it is important to note that some solution-based protocols for preparing luminescent silicon quantum dots actually yield carbon quantum dots instead of the reported silicon. The unique properties of silicon quantum dots lend themselves to an array of potential applications: biological imaging, luminescent solar concentrators, light emitting diodes, sensors, and lithium-ion battery anodes.

Dextran drug delivery systems involve the use of the natural glucose polymer dextran in applications as a prodrug, nanoparticle, microsphere, micelle, and hydrogel drug carrier in the field of targeted and controlled drug delivery. According to several in vitro and animal research studies, dextran carriers reduce off-site toxicity and improve local drug concentration at the target tissue site. This technology has significant implications as a potential strategy for delivering therapeutics to treat cancer, cardiovascular diseases, pulmonary diseases, bone diseases, liver diseases, colonic diseases, infections, and HIV.

























