
A pandemic is an epidemic of an infectious disease that has a sudden increase in cases and spreads across a large region, for instance multiple continents or worldwide, affecting a substantial number of individuals. Widespread endemic diseases with a stable number of infected individuals such as recurrences of seasonal influenza are generally excluded as they occur simultaneously in large regions of the globe rather than being spread worldwide.
A zoonosis or zoonotic disease is an infectious disease of humans caused by a pathogen that can jump from a non-human vertebrate to a human. When humans infect non-humans, it is called reverse zoonosis or anthroponosis.

Influenza A virus (IAV) is the only species of the genus Alphainfluenzavirus of the virus family Orthomyxoviridae. It is a pathogen with strains that infect birds and some mammals, as well as causing seasonal flu in humans. Mammals in which different strains of IAV circulate with sustained transmission are bats, pigs, horses and dogs; other mammals can occasionally become infected.

In infectious disease ecology and epidemiology, a natural reservoir, also known as a disease reservoir or a reservoir of infection, is the population of organisms or the specific environment in which an infectious pathogen naturally lives and reproduces, or upon which the pathogen primarily depends for its survival. A reservoir is usually a living host of a certain species, such as an animal or a plant, inside of which a pathogen survives, often without causing disease for the reservoir itself. By some definitions a reservoir may also be an environment external to an organism, such as a volume of contaminated air or water.

Nipah virus is a bat-borne, zoonotic virus that causes Nipah virus infection in humans and other animals, a disease with a very high mortality rate (40-75%). Numerous disease outbreaks caused by Nipah virus have occurred in South East Africa and Southeast Asia. Nipah virus belongs to the genus Henipavirus along with the Hendra virus, which has also caused disease outbreaks.
EcoHealth Alliance is a US-based non-governmental organization with a stated mission of protecting people, animals, and the environment from emerging infectious diseases. The nonprofit organization focuses on research aimed at preventing pandemics and promoting conservation in hotspot regions worldwide.
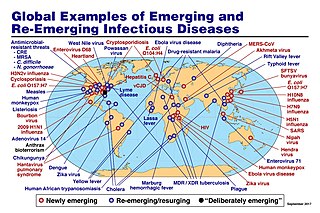
An emerging infectious disease (EID) is an infectious disease whose incidence has increased recently, and could increase in the near future. The minority that are capable of developing efficient transmission between humans can become major public and global concerns as potential causes of epidemics or pandemics. Their many impacts can be economic and societal, as well as clinical. EIDs have been increasing steadily since at least 1940.
An emergent virus is a virus that is either newly appeared, notably increasing in incidence/geographic range or has the potential to increase in the near future. Emergent viruses are a leading cause of emerging infectious diseases and raise public health challenges globally, given their potential to cause outbreaks of disease which can lead to epidemics and pandemics. As well as causing disease, emergent viruses can also have severe economic implications. Recent examples include the SARS-related coronaviruses, which have caused the 2002–2004 outbreak of SARS (SARS-CoV-1) and the 2019–2023 pandemic of COVID-19 (SARS-CoV-2). Other examples include the human immunodeficiency virus, which causes HIV/AIDS; the viruses responsible for Ebola; the H5N1 influenza virus responsible for avian influenza; and H1N1/09, which caused the 2009 swine flu pandemic. Viral emergence in humans is often a consequence of zoonosis, which involves a cross-species jump of a viral disease into humans from other animals. As zoonotic viruses exist in animal reservoirs, they are much more difficult to eradicate and can therefore establish persistent infections in human populations.

Walter Ian Lipkin is the John Snow Professor of Epidemiology at the Mailman School of Public Health at Columbia University and a professor of Neurology and Pathology at the College of Physicians and Surgeons at Columbia University. He is also director of the Center for Infection and Immunity, an academic laboratory for microbe hunting in acute and chronic diseases. Lipkin is internationally recognized for his work with West Nile virus, SARS and COVID-19.

Betacoronavirus cameli, or EMC/2012 (HCoV-EMC/2012), is the virus that causes Middle East respiratory syndrome (MERS). It is a species of coronavirus which infects humans, bats, and camels. The infecting virus is an enveloped, positive-sense, single-stranded RNA virus which enters its host cell by binding to the DPP4 receptor. The species is a member of the genus Betacoronavirus and subgenus Merbecovirus.
Spillover infection, also known as pathogen spillover and spillover event, occurs when a reservoir population with a high pathogen prevalence comes into contact with a novel host population. The pathogen is transmitted from the reservoir population and may or may not be transmitted within the host population. Due to climate change and land use expansion, the risk of viral spillover is predicted to significantly increase.
Cross-species transmission (CST), also called interspecies transmission, host jump, or spillover, is the transmission of an infectious pathogen, such as a virus, between hosts belonging to different species. Once introduced into an individual of a new host species, the pathogen may cause disease for the new host and/or acquire the ability to infect other individuals of the same species, allowing it to spread through the new host population. The phenomenon is most commonly studied in virology, but cross-species transmission may also occur with bacterial pathogens or other types of microorganisms.

The bat virome is the group of viruses associated with bats. Bats host a diverse array of viruses, including all seven types described by the Baltimore classification system: (I) double-stranded DNA viruses; (II) single-stranded DNA viruses; (III) double-stranded RNA viruses; (IV) positive-sense single-stranded RNA viruses; (V) negative-sense single-stranded RNA viruses; (VI) positive-sense single-stranded RNA viruses that replicate through a DNA intermediate; and (VII) double-stranded DNA viruses that replicate through a single-stranded RNA intermediate. The greatest share of bat-associated viruses identified as of 2020 are of type IV, in the family Coronaviridae.

Nipah virus infection is an infection caused by the Nipah virus. Symptoms from infection vary from none to fever, cough, headache, shortness of breath, and confusion. This may worsen into a coma over a day or two, and 50% to 75% of those infected die. Complications can include inflammation of the brain and seizures following recovery.

Wildlife trafficking practices have resulted in the emergence of zoonotic diseases. Exotic wildlife trafficking is a multi-billion dollar industry that involves the removal and shipment of mammals, reptiles, amphibians, invertebrates, and fish all over the world. Traded wild animals are used for bushmeat consumption, unconventional exotic pets, animal skin clothing accessories, home trophy decorations, privately owned zoos, and for traditional medicine practices. Dating back centuries, people from Africa, Asia, Latin America, the Middle East, and Europe have used animal bones, horns, or organs for their believed healing effects on the human body. Wild tigers, rhinos, elephants, pangolins, and certain reptile species are acquired through legal and illegal trade operations in order to continue these historic cultural healing practices. Within the last decade nearly 975 different wild animal taxa groups have been legally and illegally exported out of Africa and imported into areas like China, Japan, Indonesia, the United States, Russia, Europe, and South America.
Ghanaian bat henipavirus (also known Kumasi virus belongs to the genus Henipavirus in the family Paramyxoviridae. Human infections are caused by zoonotic events where the virus crosses over from another animal species. Therefore, humans are not the innate host for this virus family but instead become infected by peripheral viral reservoirs such as bats and other carriers of the virus. When these virus are spread to humans through zoonotic events they have been found to be one of the most deadly viruses with the capability to infect humans, with mortality rates between 50 and 100%. Therefore, these viruses have been classified as a biosafety level four virus with regards to its pathogenesis when it infects humans.
Pandemic prevention is the organization and management of preventive measures against pandemics. Those include measures to reduce causes of new infectious diseases and measures to prevent outbreaks and epidemics from becoming pandemics.

Peter Daszak is a British zoologist, consultant and public expert on disease ecology, in particular on zoonosis. He is the president of EcoHealth Alliance, a nonprofit non-governmental organization that supports various programs on global health and pandemic prevention. He is also a member of the Center for Infection and Immunity at the Columbia University Mailman School of Public Health. He lives in Suffern, New York.

Since the beginning of the COVID-19 pandemic, there have been efforts by scientists, governments, and others to determine the origin of the SARS-CoV-2 virus. Similar to other outbreaks, the virus was derived from a bat-borne virus and most likely was transmitted to humans via another animal in nature, or during wildlife trade such as that in food markets. While other explanations, such as speculations that SARS-CoV-2 was accidentally released from a laboratory have been proposed, such explanations are not supported by evidence. Conspiracy theories about the virus's origin have also proliferated.

The COVID-19 lab leak theory, or lab leak hypothesis, is the idea that SARS-CoV-2, the virus that caused the COVID-19 pandemic, came from a laboratory. This claim is highly controversial; most scientists believe the virus spilled into human populations through natural zoonosis, similar to the SARS-CoV-1 and MERS-CoV outbreaks, and consistent with other pandemics in human history. Available evidence suggests that the SARS-CoV-2 virus was originally harbored by bats, and spread to humans from infected wild animals, functioning as an intermediate host, at the Huanan Seafood Market in Wuhan, Hubei, China, in December 2019. Several candidate animal species have been identified as potential intermediate hosts. There is no evidence SARS-CoV-2 existed in any laboratory prior to the pandemic, or that any suspicious biosecurity incidents happened in any laboratory.













