
Morphine is a strong opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies. It is mainly used as an analgesic. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle, injection under the skin, or injection into the spinal cord area; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are available as MS-Contin, Kadian, and other brand names as well as generically.
Endorphins are peptides produced in the brain that block the perception of pain and increase feelings of wellbeing. They are produced and stored in the pituitary gland of the brain. Endorphins are endogenous painkillers often produced in the brain and adrenal medulla during physical exercise or orgasm and inhibit pain, muscle cramps, and relieve stress.
Colloquially known as "downers," depressants, or central depressants, are drugs that lower neurotransmission levels, or depress or reduce arousal or stimulation in various areas of the brain. Depressants do not change the mood or mental state of others. Stimulants, or "uppers," increase mental or physical function, hence the opposite drug class from depressants are stimulants, not antidepressants.

Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work in the brain to produce a variety of effects, including pain relief. As a class of substances, they act on opioid receptors to produce morphine-like effects.

β-Carboline (9H-pyrido[3,4-b]indole) represents the basic chemical structure for more than one hundred alkaloids and synthetic compounds. The effects of these substances depend on their respective substituent. Natural β-carbolines primarily influence brain functions but can also exhibit antioxidant effects. Synthetically designed β-carboline derivatives have recently been shown to have neuroprotective, cognitive enhancing and anti-cancer properties.

Buprenorphine, sold under the brand name Subutex among others, is an opioid used to treat opioid use disorder, acute pain, and chronic pain. It can be used under the tongue (sublingual), in the cheek (buccal), by injection, as a skin patch (transdermal), or as an implant. For opioid use disorder, it is typically started when withdrawal symptoms have begun and for the first two days of treatment under direct observation of a health-care provider.

Unani or Yunani medicine is Perso-Arabic traditional medicine as practiced in Muslim culture in South Asia and modern day Central Asia. Unani medicine is pseudoscientific. The Indian Medical Association describes Unani practitioners who claim to practice medicine as quacks.
Rat Park was a series of studies into drug addiction conducted in the late 1970s and published between 1978 and 1981 by Canadian psychologist Bruce K. Alexander and his colleagues at Simon Fraser University in British Columbia, Canada.

Ibogaine is a naturally occurring psychoactive substance found in plants in the family Apocynaceae such as Tabernanthe iboga, Voacanga africana, and Tabernaemontana undulata. It is a psychedelic with dissociative properties.

18-Methoxycoronaridine, also known as zolunicant, is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proved to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.
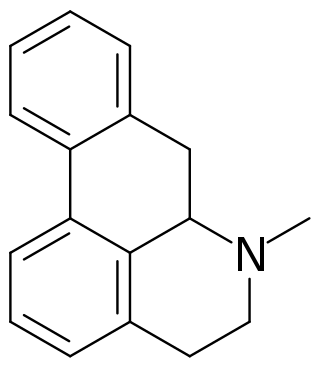
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Methyllycaconitine (MLA) is a diterpenoid alkaloid found in many species of Delphinium (larkspurs). In common with many other diterpenoid alkaloids, it is toxic to animals, although the acute toxicity varies with species. Early research was focused on identifying, and characterizing the properties of methyllycaconitine as one of the principal toxins in larkspurs responsible for livestock poisoning in the mountain rangelands of North America. Methyllycaconitine has been explored as a possible therapeutic agent for the treatment of spastic paralysis, and it has been shown to have insecticidal properties. Most recently, it has become an important molecular probe for studying the pharmacology of the nicotinic acetylcholine receptor.

Lobeline is a piperidine alkaloid found in a variety of plants, particularly those in the genus Lobelia, including Indian tobacco, Devil's tobacco, great lobelia, Lobelia chinensis, and Hippobroma longiflora. In its pure form, it is a white amorphous powder which is freely soluble in water.

Delphinine is a toxic diterpenoid alkaloid found in plants from the Delphinium (larkspur) and Atragene genera, both in the family Ranunculaceae. Delphinine is the principal alkaloid found in Delphinium staphisagria seeds – at one time, under the name stavesacre, a very well known herbal treatment for body lice. It is related in structure and has similar effects to aconitine, acting as an allosteric modulator of voltage gated sodium channels, and producing low blood pressure, slowed heart rate and abnormal heart rhythms. These effects make it highly poisonous. While it has been used in some alternative medicines, most of the medical community does not recommend using it due to its extreme toxicity.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others.

An opiate is an alkaloid substance derived from opium It has a different meaning from the similar term opioid, used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.

Ibogamine is an anti-convulsant, anti-addictive, CNS stimulant alkaloid found in Tabernanthe iboga and Crepe Jasmine. Basic research related to how addiction affects the brain has used this chemical.

Syed Ziaur Rahman is a permanent member of 'Board of Trustees' and Chair of the Advisory Council, International Association of Medical Colleges (IAOMC). He also serves as elected secretary of IAOMC and Society of Pharmacovigilance, India (SoPI).

Mitragynine is an indole-based alkaloid and the most abundant active alkaloid in the Southeast Asian plant Mitragyna speciosa, commonly known as kratom. The total alkaloid concentration in dried leaves ranges from 0.5 to 1.5%. In Thai varieties, mitragynine is the most abundant component while 7-hydroxymitragynine is a minor constituent. In Malaysian kratom varieties, mitragynine is present at lower concentration. Such preparations are orally consumed and typically involve dried kratom leaves which are brewed into tea or ground and placed into capsules. Mitragynine consumption for medicinal and recreation purposes dates back centuries, although early use was primarily limited to Southeast Asian countries such as Indonesia and Thailand where the plant grows indigenously. Recently, mitragynine use has spread throughout Europe and the Americas as both a recreational and medicinal drug. While research into the effects of kratom have begun to emerge, investigations on the active compound mitragynine are less common.















