
Cytochromes P450 are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in Escherichia coli. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted.

Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme catalyzes sequential hydroxylations of the steroid angular methyl group at C18 after initial 11β-hydroxylation. It is encoded by the CYP11B2 gene in humans.

Cholesterol 24-hydroxylase, also commonly known as cholesterol 24S-hydroxylase, cholesterol 24-monooxygenase, CYP46, or CYP46A1, is an enzyme that catalyzes the conversion of cholesterol to 24S-hydroxycholesterol. It is responsible for the majority of cholesterol turnover in the human central nervous system. The systematic name of this enzyme class is cholesterol,NADPH:oxygen oxidoreductase (24-hydroxylating).
Ecdysone 20-monooxygenase (EC 1.14.99.22) is an enzyme that catalyzes the chemical reaction
In enzymology, a licodione synthase (EC 1.14.13.87) is an enzyme that catalyzes the chemical reaction

[Methionine synthase] reductase, or Methionine synthase reductase, encoded by the gene MTRR, is an enzyme that is responsible for the reduction of methionine synthase inside human body. This enzyme is crucial for maintaining the one carbon metabolism, specifically the folate cycle. The enzyme employs one coenzyme, flavoprotein.

In enzymology, a NADPH—hemoprotein reductase is an enzyme that catalyzes the chemical reaction

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer as well as antimicrobial drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.
5-exo-hydroxycamphor dehydrogenase (EC 1.1.1.327, F-dehydrogenase, FdeH) is an enzyme with systematic name 5-exo-hydroxycamphor:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction
Tyrosine N-monooxygenase (EC 1.14.13.41, tyrosine N-hydroxylase, CYP79A1) is an enzyme with systematic name L-tyrosine,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Flavonoid 3',5'-hydroxylase (EC 1.14.14.81 was wrongly classified as EC 1.14.13.88 in the past) is an enzyme with systematic name flavanone,NADPH:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction

(+)-Menthofuran synthase (EC 1.14.13.104, menthofuran synthase, (+)-pulegone 9-hydroxylase, (+)-MFS, cytochrome P450 menthofuran synthase) is an enzyme with systematic name (+)-pulegone,NADPH:oxygen oxidoreductase (9-hydroxylating). This enzyme catalyses the following chemical reaction
Abieta-7,13-dien-18-ol hydroxylase (EC 1.14.13.109, CYP720B1, PTAO) is an enzyme with systematic name abieta-7,13-dien-18-ol,NADPH:oxygen oxidoreductase (18-hydroxylating). This enzyme catalyses the following chemical reaction
Cholest-4-en-3-one 26-monooxygenase (EC 1.14.13.141, CYP125, CYP125A1, cholest-4-en-3-one 27-monooxygenase) is an enzyme with systematic name cholest-4-en-3-one,NADH:oxygen oxidoreductase (26-hydroxylating). This enzyme catalyses the following chemical reaction
Nitric-oxide synthase (NAD(P)H-dependent) (EC 1.14.14.47, nitric oxide synthetase, NO synthase) is an enzyme with systematic name L-arginine,NAD(P)H:oxygen oxidoreductase (nitric-oxide-forming). This enzyme catalyses the following chemical reaction
5,6-dimethylbenzimidazole synthase (EC 1.14.99.40, BluB) is an enzyme with systematic name FMNH2 oxidoreductase (5,6-dimethylbenzimidazole forming). This enzyme catalyses the following chemical reaction
Deoxyhypusine synthase (EC 2.5.1.46, spermidine:eIF5A-lysine 4-aminobutyltransferase (propane-1,3-diamine-forming)) is an enzyme with systematic name (eIF5A-precursor)-lysine:spermidine 4-aminobutyltransferase (propane-1,3-diamine-forming). This enzyme catalyses the following chemical reaction
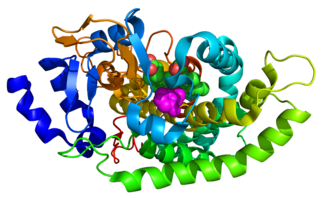
Cytochrome P450 aromatic O-demethylase is a bacterial enzyme that catalyzes the demethylation of lignin and various lignols. The net reaction follows the following stoichiometry, illustrated with a generic methoxy arene:
CYP6M2 is a gene location in Anopheles gambiae chromosome 3R, involved in the insecticide resistant. The enzyme encoded by this gene is capable of directly metabolizing pyrethroids, belongs to the cytochrome P450 family CYP6

Bettie Sue Siler Masters is an adjunct professor at Duke University known for her work on nitric oxide synthase and cytochrome P450 reductase. She was the 1992 recipient of the FASEB Excellence in Science Award, and has been elected as a member of the National Academy of Medicine and as a fellow of the American Association for the Advancement of Science.









