
Osmium is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element, with an experimentally measured density of 22.59 g/cm3. Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen nib tipping, electrical contacts, and in other applications that require extreme durability and hardness. The element's abundance in the Earth's crust is among the rarest.

In chemistry, a nonmetal is a chemical element that mostly lacks the characteristics of a metal. Physically, a nonmetal tends to have a relatively low melting point, boiling point, and density. A nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity. Chemically, nonmetals tend to have relatively high ionization energy, electron affinity, and electronegativity. They gain or share electrons when they react with other elements and chemical compounds. Seventeen elements are generally classified as nonmetals: most are gases ; one is a liquid (bromine); and a few are solids. Metalloids such as boron, silicon, and germanium are sometimes counted as nonmetals.

In chemistry, an oxidizing agent is a substance that has the ability to oxidize other substances — in other words to accept their electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C (-33 F°); above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).

Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen.
Molybdenum trioxide is chemical compound with the formula MoO3. This compound is produced on the largest scale of any molybdenum compound. It is an intermediate in the production of molybdenum metal. It is also an important industrial catalyst. Molybdenum trioxide occurs as the rare mineral molybdite.

Arsenic trioxide is an inorganic compound with the formula As
2O
3. This commercially important oxide of arsenic is the main precursor to other arsenic compounds, including organoarsenic compounds. Approximately 50,000 tonnes are produced annually. Many applications are controversial given the high toxicity of arsenic compounds.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis.

Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate.
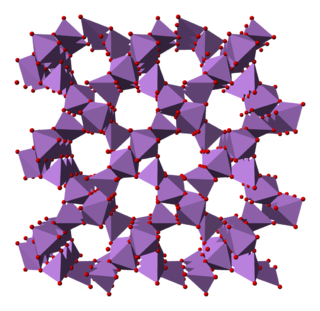
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All arsenic compounds are highly toxic and thus find only limited commercial applications.

Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.

Gallium(III) trioxide is an inorganic compound with the formula Ga2O3. It exists as several polymorphs, all of which are white, water-insoluble solids. Although no commercial applications exist, Ga2O3 is an intermediate in the purification of gallium, which is consumed almost exclusively as gallium arsenide.

Rhenium trioxide or rhenium(VI) oxide is an inorganic compound with the formula ReO3. It is a red solid with a metallic lustre, which resembles copper in appearance. It is the only stable trioxide of the Group 7 elements (Mn, Tc, Re).

Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. It has been used as a reducing agent in organic chemistry. It is a rare example of a compound with phosphorus in the +2 oxidation state, and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides.
Phosphorus oxide can refer to:

Dinitrogen trioxide is the chemical compound with the formula N2O3. This deep blue solid is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):



















