Related Research Articles

Leukemia is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called blasts or leukemia cells. Symptoms may include bleeding and bruising,bone pain,fatigue,fever,and an increased risk of infections. These symptoms occur due to a lack of normal blood cells. Diagnosis is typically made by blood tests or bone marrow biopsy.

The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells. This chromosome is defective and unusually short because of reciprocal translocation,t(9;22)(q34;q11),of genetic material between chromosome 9 and chromosome 22,and contains a fusion gene called BCR-ABL1. This gene is the ABL1 gene of chromosome 9 juxtaposed onto the breakpoint cluster region BCR gene of chromosome 22,coding for a hybrid protein:a tyrosine kinase signaling protein that is "always on",causing the cell to divide uncontrollably by interrupting the stability of the genome and impairing various signaling pathways governing the cell cycle.

Chronic myelogenous leukemia (CML),also known as chronic myeloid leukemia,is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes and their precursors is found;characteristic increase in basophils is clinically relevant. It is a type of myeloproliferative neoplasm associated with a characteristic chromosomal translocation called the Philadelphia chromosome.

Imatinib,sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others,is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple tyrosine kinases such as CSF1R,ABL,c-KIT,FLT3,and PDGFR-β. Specifically,it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome–positive (Ph+),certain types of gastrointestinal stromal tumors (GIST),hypereosinophilic syndrome (HES),chronic eosinophilic leukemia (CEL),systemic mastocytosis,and myelodysplastic syndrome.

Daunorubicin,also known as daunomycin,is a chemotherapy medication used to treat cancer. Specifically it is used for acute myeloid leukemia (AML),acute lymphoblastic leukemia (ALL),chronic myelogenous leukemia (CML),and Kaposi's sarcoma. It is administered by injection into a vein. A liposomal formulation known as liposomal daunorubicin also exists.
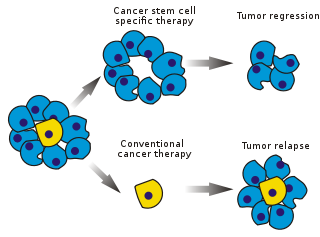
Cancer stem cells (CSCs) are cancer cells that possess characteristics associated with normal stem cells,specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming),perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are hypothesized to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore,development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients,especially for patients with metastatic disease.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer,others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine,targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth,rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals,the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However,the modalities can be combined;antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
K562 cells were the first human immortalised myelogenous leukemia cell line to be established. K562 cells are of the erythroleukemia type,and the cell line is derived from a 53-year-old female chronic myelogenous leukemia patient in blast crisis. The cells are non-adherent and rounded,are positive for the bcr:abl fusion gene,and bear some proteomic resemblance to both undifferentiated granulocytes and erythrocytes.
Systems immunology is a research field under systems biology that uses mathematical approaches and computational methods to examine the interactions within cellular and molecular networks of the immune system. The immune system has been thoroughly analyzed as regards to its components and function by using a "reductionist" approach,but its overall function can't be easily predicted by studying the characteristics of its isolated components because they strongly rely on the interactions among these numerous constituents. It focuses on in silico experiments rather than in vivo.

Raúl Rabadán is a Spanish-American theoretical physicist and computational biologist. He is currently the Gerald and Janet Carrus Professor in the Department of Systems Biology,Biomedical Informatics and Surgery at Columbia University. He is the director of the Program for Mathematical Genomics at Columbia University and director of the Center for Topology of Cancer Evolution and Heterogeneity. At Columbia,he has put together a highly interdisciplinary lab with researchers from the fields of mathematics,physics,computer science,engineering,and medicine,with the common goal of solving pressing biomedical problems through quantitative computational models. Rabadan's current interest focuses on uncovering patterns of evolution in biological systems—in particular,viruses and cancer.

Acute myeloblastic leukemia with maturation (M2) is a subtype of acute myeloid leukemia (AML).

Tyrosine-protein kinase receptor UFO is an enzyme that in humans is encoded by the AXL gene. The gene was initially designated as UFO,in allusion to the unidentified function of this protein. However,in the years since its discovery,research into AXL's expression profile and mechanism has made it an increasingly attractive target,especially for cancer therapeutics. In recent years,AXL has emerged as a key facilitator of immune escape and drug-resistance by cancer cells,leading to aggressive and metastatic cancers.
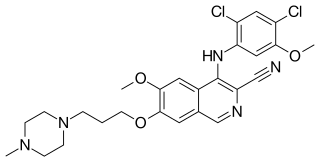
Bosutinib,sold under the brand name Bosulif,is a small molecule BCR-ABL and src tyrosine kinase inhibitor used for the treatment of chronic myelogenous leukemia.
Somatic evolution is the accumulation of mutations and epimutations in somatic cells during a lifetime,and the effects of those mutations and epimutations on the fitness of those cells. This evolutionary process has first been shown by the studies of Bert Vogelstein in colon cancer. Somatic evolution is important in the process of aging as well as the development of some diseases,including cancer.
Cancer cells are cells that divide continually,forming solid tumors or flooding the blood or lymph with abnormal cells. Cell division is a normal process used by the body for growth and repair. A parent cell divides to form two daughter cells,and these daughter cells are used to build new tissue or to replace cells that have died because of aging or damage. Healthy cells stop dividing when there is no longer a need for more daughter cells,but cancer cells continue to produce copies. They are also able to spread from one part of the body to another in a process known as metastasis.

Ponatinib,sold under the brand name Iclusig,is a medication used for the treatment of chronic myeloid leukemia and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia. It was developed by Ariad Pharmaceuticals. It is a multi-targeted tyrosine-kinase inhibitor. Some forms of chronic myeloid leukemia,those that have the T315I mutation,are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.
Antineoplastic resistance,often used interchangeably with chemotherapy resistance,is the resistance of neoplastic (cancerous) cells,or the ability of cancer cells to survive and grow despite anti-cancer therapies. In some cases,cancers can evolve resistance to multiple drugs,called multiple drug resistance.
Tumour heterogeneity describes the observation that different tumour cells can show distinct morphological and phenotypic profiles,including cellular morphology,gene expression,metabolism,motility,proliferation,and metastatic potential. This phenomenon occurs both between tumours and within tumours. A minimal level of intra-tumour heterogeneity is a simple consequence of the imperfection of DNA replication:whenever a cell divides,a few mutations are acquired—leading to a diverse population of cancer cells. The heterogeneity of cancer cells introduces significant challenges in designing effective treatment strategies. However,research into understanding and characterizing heterogeneity can allow for a better understanding of the causes and progression of disease. In turn,this has the potential to guide the creation of more refined treatment strategies that incorporate knowledge of heterogeneity to yield higher efficacy.
Patient derived xenografts (PDX) are models of cancer where the tissue or cells from a patient's tumor are implanted into an immunodeficient or humanized mouse. It is a form of xenotransplantation. PDX models are used to create an environment that allows for the continued growth of cancer after its removal from a patient. In this way,tumor growth can be monitored in the laboratory,including in response to potential therapeutic options. Cohorts of PDX models can be used to determine the therapeutic efficiency of a therapy against particular types of cancer,or a PDX model from a specific patient can be tested against a range of therapies in a 'personalized oncology' approach.
Franziska Michor is an Austrian computational biologist. She is a professor in the department of data science at the Dana–Farber Cancer Institute. She serves as Director of the Physical Sciences-Oncology Center and the Center for Cancer Evolution.
References
- 1 2 3 4 "Dr. Doron Levy, PhD – Editorial Board – ImmunoInformatics – Journal – Elsevier". www.journals.elsevier.com.
- 1 2 "MRC Director". Brin MRC.
- 1 2 "NSF Award Search: Award # 0820817 – Career: Partial Differential Equation-based Image Processing with Applications to Radiation Oncology". www.nsf.gov.
- 1 2 "Doron Levy". John Simon Guggenheim Memorial Foundation...
- ↑ "Bulletin of Mathematical Biology – Editors".
- ↑ "Editorial Team – Le Matematiche".
- ↑ "Editors – Acta Applicandae Mathematicae".
- ↑ "Editors – Cancer Research – American Association for Cancer Research".
- ↑ "Editorial board – Applied Mathematical Modelling – ScienceDirect.com by Elsevier".
- ↑ "Editorial Board – PLOS ONE".
- ↑ "Differential Equations and Dynamical Systems – Editors".
- ↑ Levy, Doron; Tadmor, Eitan (January 11, 1998). "From Semidiscrete to Fully Discrete: Stability of Runge—Kutta Schemes by The Energy Method". SIAM Review. 40 (1): 40–73. Bibcode:1998SIAMR..40...40L. doi:10.1137/S0036144597316255. hdl: 1903/8644 – via CrossRef.
- ↑ "Wolfgang Pauli Institute (WPI)". www.wpi.ac.at.
- ↑ "Department of Mathematics – Levy, Doron". www-math.umd.edu.
- ↑ Levy, Doron; Puppo, Gabriella; Russo, Giovanni (May 1, 2000). "On the behavior of the total variation in CWENO methods for conservation laws". Applied Numerical Mathematics. 33 (1): 407–414. doi:10.1016/S0168-9274(99)00107-5 – via ScienceDirect.
- ↑ Levy, Doron; Puppo, Gabriella; Russo, Giovanni (January 11, 2000). "Compact Central WENO Schemes for Multidimensional Conservation Laws". SIAM Journal on Scientific Computing. 22 (2): 656–672. arXiv: math/9911089 . Bibcode:2000SJSC...22..656L. doi:10.1137/S1064827599359461. S2CID 12442855 – via CrossRef.
- ↑ Levy, Doron; Puppo, Gabriella; Russo, Giovanni (May 1, 2000). "A third order central WENO scheme for 2D conservation laws". Applied Numerical Mathematics. 33 (1): 415–421. doi:10.1016/S0168-9274(99)00108-7 – via ScienceDirect.
- ↑ Bryson, S.; Levy, D. (August 11, 2003). "High-order central WENO schemes for 1D Hamilton-Jacobi equations". In Brezzi, Franco; Buffa, Annalisa; Corsaro, Stefania; Murli, Almerico (eds.). Numerical Mathematics and Advanced Applications. Springer Milan. pp. 45–54. doi:10.1007/978-88-470-2089-4_4. hdl:2060/20020059594. ISBN 978-88-470-2167-9 – via Springer Link.
- ↑ Bryson, Steve; Levy, Doron (January 11, 2003). "High-Order Central WENO Schemes for Multidimensional Hamilton-Jacobi Equations". SIAM Journal on Numerical Analysis. 41 (4): 1339–1369. doi:10.1137/S0036142902408404. hdl: 2060/20020066776 . S2CID 5842936 – via CrossRef.
- ↑ Levy, Doron; Nayak, Suhas; Shu, Chi-Wang; Zhang, Yong-Tao (January 11, 2006). "Central WENO Schemes for Hamilton–Jacobi Equations on Triangular Meshes". SIAM Journal on Scientific Computing. 28 (6): 2229–2247. Bibcode:2006SJSC...28.2229L. doi:10.1137/040612002 – via CrossRef.
- ↑ Peet, M. M.; Kim, P. S.; Niculescu, S.-I.; Levy, D. (January 11, 2009). "New Computational Tools for Modeling Chronic Myelogenous Leukemia". Mathematical Modelling of Natural Phenomena. 4 (2): 119–139. doi: 10.1051/mmnp/20094206 .
- ↑ Botesteanu, Dana-Adriana; Lipkowitz, Stanley; Lee, Jung-Min; Levy, Doron (July 11, 2016). "Mathematical models of breast and ovarian cancers". WIREs Systems Biology and Medicine. 8 (4): 337–362. doi:10.1002/wsbm.1343. PMC 4911289 . PMID 27259061.
- ↑ Botesteanu, Dana-Adriana; Lee, Jung-Min; Levy, Doron (June 3, 2016). "Modeling the Dynamics of High-Grade Serous Ovarian Cancer Progression for Transvaginal Ultrasound-Based Screening and Early Detection". PLOS ONE. 11 (6): e0156661. Bibcode:2016PLoSO..1156661B. doi: 10.1371/journal.pone.0156661 . PMC 4892570 . PMID 27257824.
- ↑ Wilson, Shelby; Levy, Doron (October 1, 2013). "Functional Switching and Stability of Regulatory T Cells". Bulletin of Mathematical Biology. 75 (10): 1891–1911. doi:10.1007/s11538-013-9875-9. PMID 23917986. S2CID 255314821 – via Springer Link.
- ↑ Greene, James M.; Levy, Doron; Fung, King Leung; Souza, Paloma S.; Gottesman, Michael M.; Lavi, Orit (February 21, 2015). "Modeling intrinsic heterogeneity and growth of cancer cells". Journal of Theoretical Biology. 367: 262–277. doi:10.1016/j.jtbi.2014.11.017. PMC 4308514 . PMID 25457229.
- ↑ Lorz, Alexander; Botesteanu, Dana-Adriana; Levy, Doron (August 11, 2017). "Modeling Cancer Cell Growth Dynamics In vitro in Response to Antimitotic Drug Treatment". Frontiers in Oncology. 7: 189. doi: 10.3389/fonc.2017.00189 . PMC 5582072 . PMID 28913178.
- ↑ Milzman, Jesse; Sheng, Wanqiang; Levy, Doron (January 12, 2021). "Modeling LSD1-Mediated Tumor Stagnation". Bulletin of Mathematical Biology. 83 (2): 15. doi:10.1007/s11538-020-00842-8. PMID 33433736. S2CID 231586558 – via Springer Link.
- ↑ Besse, Apollos; Clapp, Geoffrey D.; Bernard, Samuel; Nicolini, Franck E.; Levy, Doron; Lepoutre, Thomas (May 1, 2018). "Stability Analysis of a Model of Interaction Between the Immune System and Cancer Cells in Chronic Myelogenous Leukemia". Bulletin of Mathematical Biology. 80 (5): 1084–1110. doi:10.1007/s11538-017-0272-7. PMID 28536994. S2CID 4036621 – via Springer Link.
- ↑ "The role of the autologous immune response in chronic myelogenous leukemia".
- ↑ Clapp, Geoffrey D.; Lepoutre, Thomas; Nicolini, Franck E.; Levy, Doron (May 3, 2016). "BCR-ABL transcript variations in chronic phase chronic myelogenous leukemia patients on imatinib first-line: Possible role of the autologous immune system". OncoImmunology. 5 (5): e1122159. doi:10.1080/2162402X.2015.1122159. PMC 4910749 . PMID 27467931.
- ↑ Lavi, Orit; Gottesman, Michael M.; Levy, Doron (February 1, 2012). "The dynamics of drug resistance: A mathematical perspective". Drug Resistance Updates. 15 (1): 90–97. doi:10.1016/j.drup.2012.01.003. PMC 3348255 . PMID 22387162.
- ↑ Tomasetti, C.; Levy, D. (2010). "An Elementary Approach to Modeling Drug Resistance in Cancer". Mathematical Biosciences and Engineering. 7 (4): 905–918. doi:10.3934/mbe.2010.7.905. PMC 3877932 . PMID 21077714.
- ↑ Tomasetti, C.; Levy, D. (August 11, 2010). "Drug Resistance always Depends on the Turnover Rate". In Herold, Keith E.; Vossoughi, Jafar; Bentley, William E. (eds.). 26th Southern Biomedical Engineering Conference SBEC 2010, April 30 - May 2, 2010, College Park, Maryland, USA. IFMBE Proceedings. Vol. 32. Springer. pp. 552–555. doi:10.1007/978-3-642-14998-6_141. ISBN 978-3-642-14997-9 – via Springer Link.
- ↑ Cho, Heyrim; Levy, Doron (December 1, 2017). "Modeling the Dynamics of Heterogeneity of Solid Tumors in Response to Chemotherapy". Bulletin of Mathematical Biology. 79 (12): 2986–3012. doi:10.1007/s11538-017-0359-1. PMID 29022203. S2CID 41563051 – via Springer Link.
- ↑ Becker, Matthew; Levy, Doron (October 1, 2017). "Modeling the Transfer of Drug Resistance in Solid Tumors". Bulletin of Mathematical Biology. 79 (10): 2394–2412. doi:10.1007/s11538-017-0334-x. hdl: 1903/20883 . PMID 28852953. S2CID 255318869 – via Springer Link.
- ↑ Cho, Heyrim; Wang, Zuping; Levy, Doron (November 21, 2020). "Study of dose-dependent combination immunotherapy using engineered T cells and IL-2 in cervical cancer". Journal of Theoretical Biology. 505: 110403. arXiv: 2001.10573 . Bibcode:2020JThBi.50510403C. doi:10.1016/j.jtbi.2020.110403. PMID 32693004. S2CID 210942624 – via ScienceDirect.
- ↑ "Mathematical Modeling Reveals That Changes to Local Cell Density Dynamically Modulate Baseline Variations in Cell Growth and Drug Response".
- ↑ "The Role of Cell Density and Intratumoral Heterogeneity in Multidrug Resistance".
- ↑ Greene, James; Lavi, Orit; Gottesman, Michael M.; Levy, Doron (March 1, 2014). "The Impact of Cell Density and Mutations in a Model of Multidrug Resistance in Solid Tumors". Bulletin of Mathematical Biology. 76 (3): 627–653. doi:10.1007/s11538-014-9936-8. PMC 4794109 . PMID 24553772 – via Springer Link.
- ↑ "Simplifying the complexity of resistance heterogeneity in metastasis: Trends in Molecular Medicine".
- ↑ Cho, Heyrim; Levy, Doron (December 1, 2018). "Modeling continuous levels of resistance to multidrug therapy in cancer". Applied Mathematical Modelling. 64: 733–751. arXiv: 1806.07557 . doi:10.1016/j.apm.2018.07.025. S2CID 49322143 – via ScienceDirect.
- ↑ Cho, Heyrim; Levy, Doron (August 11, 2020). "The impact of competition between cancer cells and healthy cells on optimal drug delivery". Mathematical Modelling of Natural Phenomena. 15: 42. arXiv: 1806.07477 . doi: 10.1051/mmnp/2019043 – via www.mmnp-journal.org.
- ↑ "Guggenheim Fellow - Doron Levy".
- ↑ Samuel Patrick Smith, ed. "The linking ring Vol 100 Issue 10", October 2020, p. 60
- ↑ "2024 Class of Fellows of the AMS". American Mathematical Society. Retrieved 2023-11-09.