
gamma-Hydroxybutyric acid (GHB), also known as 4-hydroxybutanoic acid is a naturally occurring neurotransmitter and a depressant drug. It is a precursor to GABA, glutamate, and glycine in certain brain areas. It acts on the GHB receptor and is a weak agonist at the GABAB receptor. GHB has been used in the medical setting as a general anesthetic and as treatment for cataplexy, narcolepsy, and alcoholism. The substance is also used illicitly for various reasons, including as a performance-enhancing drug, date rape drug, and as a recreational drug.

Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG.
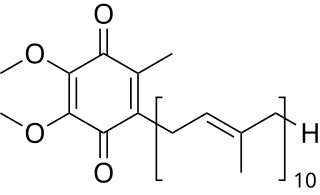
Coenzyme Q10 (CoQ10) also known as ubiquinone, is a naturally occurring biochemical cofactor (coenzyme) and an antioxidant produced by the human body. It can also be obtained from dietary sources, such as meat, fish, seed oils, vegetables, and dietary supplements. CoQ10 is found in many organisms, including animals and bacteria.

Myalgia or muscle pain is a painful sensation evolving from muscle tissue. It is a symptom of many diseases. The most common cause of acute myalgia is the overuse of a muscle or group of muscles; another likely cause is viral infection, especially when there has been no injury.

Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. Along with mast cells and basophils, they also control mechanisms associated with allergy and asthma. They are granulocytes that develop during hematopoiesis in the bone marrow before migrating into blood, after which they are terminally differentiated and do not multiply.

Eosinophilia is a condition in which the eosinophil count in the peripheral blood exceeds 5×108/L (500/μL). Hypereosinophilia is an elevation in an individual's circulating blood eosinophil count above 1.5 × 109/L (i.e. 1,500/μL). The hypereosinophilic syndrome is a sustained elevation in this count above 1.5 × 109/L (i.e. 1,500/μL) that is also associated with evidence of eosinophil-based tissue injury.
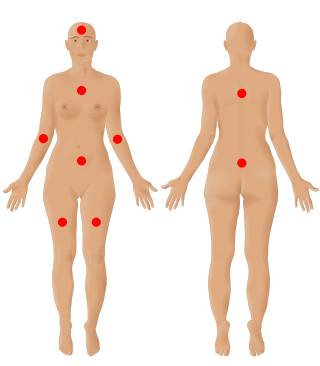
Fibromyalgia is a medical syndrome which causes chronic widespread pain, accompanied by fatigue, waking unrefreshed, and cognitive symptoms. Other symptoms can include headaches, lower abdominal pain or cramps, and depression. People with fibromyalgia can also experience insomnia and a general hypersensitivity. The cause of fibromyalgia is unknown, but is believed to involve a combination of genetic and environmental factors. Environmental factors may include psychological stress, trauma, and certain infections. Since the pain appears to result from processes in the central nervous system, the condition is referred to as a "central sensitization syndrome".

Vasculitis is a group of disorders that destroy blood vessels by inflammation. Both arteries and veins are affected. Lymphangitis is sometimes considered a type of vasculitis. Vasculitis is primarily caused by leukocyte migration and resultant damage. Although both occur in vasculitis, inflammation of veins (phlebitis) or arteries (arteritis) on their own are separate entities.

5-Hydroxytryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.

Showa Denko K. K., founded in 1939 by the merger of Nihon Electrical Industries and Showa Fertilizers, both established by a Japanese entrepreneur Nobuteru Mori, is a Japanese chemical company producing chemical products and industrial materials.
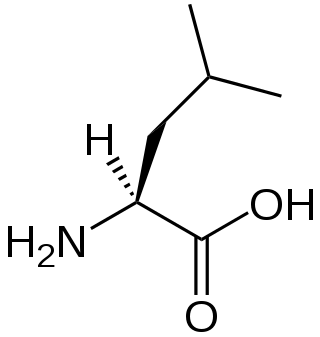
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.
Eosinophilic fasciitis, also known as Shulman's syndrome, is an inflammatory disease that affects the fascia, other connective tissues, surrounding muscles, blood vessels and nerves. Unlike other forms of fasciitis, eosinophilic fasciitis is typically self-limited and confined to the arms and legs, although it can require treatment with corticosteroids, and some cases are associated with aplastic anemia.

Toxic oil syndrome (TOS) or simply toxic syndrome is a musculoskeletal disease. A 1981 outbreak in Spain which affected about 25,600 people, with over 4,000 dying within a few months and a few thousand remaining disabled, is thought to have been caused by contaminated colza (rapeseed) oil. It was unique because of its size, the novelty of the clinical condition, and the complexity of its aetiology. Its first appearance was as a lung disease, with unusual features, though the symptoms initially resembled a lung infection. The disease appeared to be restricted to certain geographical localities, and several members of a family could be affected, even while their neighbours had no symptoms. Following the acute phase, a range of other chronic symptoms was apparent.

l-Kynurenine is a metabolite of the amino acid l-tryptophan used in the production of niacin.

Scleroderma is a group of autoimmune diseases that may result in changes to the skin, blood vessels, muscles, and internal organs. The disease can be either localized to the skin or involve other organs, as well. Symptoms may include areas of thickened skin, stiffness, feeling tired, and poor blood flow to the fingers or toes with cold exposure. One form of the condition, known as CREST syndrome, classically results in calcium deposits, Raynaud's syndrome, esophageal problems, thickening of the skin of the fingers and toes, and areas of small, dilated blood vessels.
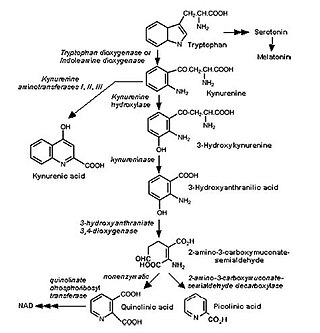
The kynurenine pathway is a metabolic pathway leading to the production of nicotinamide adenine dinucleotide (NAD+). Metabolites involved in the kynurenine pathway include tryptophan, kynurenine, kynurenic acid, xanthurenic acid, quinolinic acid, and 3-hydroxykynurenine. The kynurenine pathway is responsible for about 95% of total tryptophan catabolism. Disruption in the pathway is associated with certain genetic and psychiatric disorders.
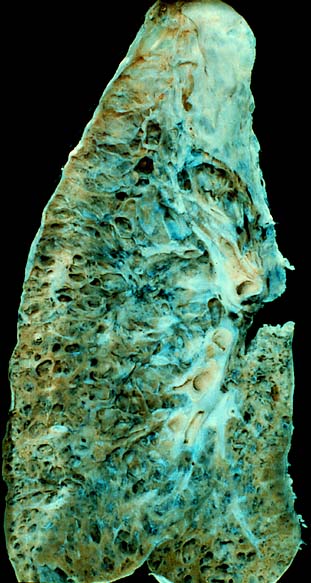
Antisynthetase syndrome (ASS) is a multisystematic autoimmune disease associated with inflammatory myositis, interstitial lung disease, and antibodies directed against various synthetases of aminoacyl-transfer RNA. Other common symptoms include mechanic's hands, Raynaud's phenomenon, arthritis, and fever.
Eosinophilic myocarditis is inflammation in the heart muscle that is caused by the infiltration and destructive activity of a type of white blood cell, the eosinophil. Typically, the disorder is associated with hypereosinophilia, i.e. an eosinophil blood cell count greater than 1,500 per microliter. It is distinguished from non-eosinophilic myocarditis, which is heart inflammation caused by other types of white blood cells, i.e. lymphocytes and monocytes, as well as the respective descendants of these cells, NK cells and macrophages. This distinction is important because the eosinophil-based disorder is due to a particular set of underlying diseases and its preferred treatments differ from those for non-eosinophilic myocarditis.
Kelly J. Henning is an epidemiologist and medical doctor currently leading the public health program of Bloomberg Philanthropies. She has led the program since it began in 2007. She was the first person to serve as director of epidemiology for the New York City Department of Health and Mental Hygiene. Henning said of working in public health "I have the opportunity to help improve the health and lives of millions of people. That's what really speaks to me."
Benign acute childhood myositis (BACM) is a syndrome characterized by muscle weakness and pain in the lower limbs that develop in children after a recent viral illness. It is transient with a spontaneous clinical resolution within 1 week.















