
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex.

Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. These proteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. The flavoproteins are some of the most-studied families of enzymes.
In enzymology, a glyoxylate reductase (NADP+) (EC 1.1.1.79) is an enzyme that catalyzes the chemical reaction
In enzymology, a tetrahydroxynaphthalene reductase (EC 1.1.1.252) is an enzyme that catalyzes the chemical reaction
In enzymology, a 1,5-anhydro-D-fructose reductase (1,5-anhydro-D-mannitol-forming) (EC 1.1.1.292) is an enzyme that catalyzes the chemical reaction

4-hydroxyphenylacetate 3-monooxygenase (EC 1.14.14.9) is an enzyme that catalyzes the chemical reaction
In enzymology, an alkanesulfonate monooxygenase (EC 1.14.14.5) is an enzyme that catalyzes the chemical reaction

In enzymology, a cob(II)yrinic acid a,c-diamide reductase is an enzyme that catalyzes the chemical reaction
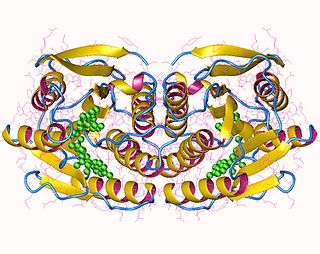
In enzymology, 6,7-dihydropteridine reductase (EC 1.5.1.34, also Dihydrobiopterin reductase) is an enzyme that catalyzes the chemical reaction
Azobenzene reductase also known as azoreductase (EC 1.7.1.6) is an enzyme that catalyzes the chemical reaction:
In enzymology, a CoA-disulfide reductase (EC 1.8.1.14) is an enzyme that catalyzes the chemical reaction
Flavin reductase a class of enzymes. There are a variety of flavin reductases, which bind free flavins and through hydrogen bonding, catalyze the reduction of these molecules to a reduced flavin. Riboflavin, or vitamin B, and flavin mononucleotide are two of the most well known flavins in the body and are used in a variety of processes which include metabolism of fat and ketones and the reduction of methemoglobin in erythrocytes. Flavin reductases are similar and often confused for ferric reductases because of their similar catalytic mechanism and structures.
In enzymology, an FMN reductase (EC 1.5.1.29) is an enzyme that catalyzes the chemical reaction
In enzymology, a mycothione reductase (EC 1.8.1.15) is an enzyme that catalyzes the chemical reaction
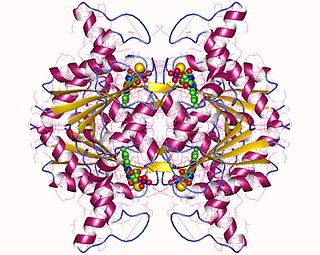
In enzymology, a NAD(P)H dehydrogenase (quinone) (EC 1.6.5.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a NADPH—hemoprotein reductase is an enzyme that catalyzes the chemical reaction
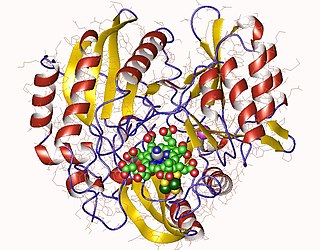
Sulfite reductase (NADPH) (EC 1.8.1.2, sulfite (reduced nicotinamide adenine dinucleotide phosphate) reductase, NADPH-sulfite reductase, NADPH-dependent sulfite reductase, H2S-NADP oxidoreductase, sulfite reductase (NADPH2)) is an enzyme with systematic name hydrogen-sulfide:NADP+ oxidoreductase. This enzyme catalises the following chemical reaction
5β-Reductase, or Δ4-3-oxosteroid 5β-reductase (EC 1.3.1.3, 3-oxo-Δ4-steroid 5β-reductase, androstenedione 5β-reductase, cholestenone 5β-reductase, cortisone 5β-reductase, cortisone Δ4-5β-reductase, steroid 5β-reductase, testosterone 5β-reductase, Δ4-3-ketosteroid 5β-reductase, Δ4-5β-reductase, Δ4-hydrogenase, 4,5β-dihydrocortisone:NADP+ Δ4-oxidoreductase, 3-oxo-5β-steroid:NADP+ Δ4-oxidoreductase) is an enzyme with systematic name 5β-cholestan-3-one:NADP+ 4,5-oxidoreductase. This enzyme catalyses the following chemical reaction
FMN reductase (NADH) (EC 1.5.1.42, NADH-FMN reductase) is an enzyme with systematic name FMNH2:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction
Morphinone reductase is an enzyme which catalyzes the NADH-dependent saturation of the carbon-carbon double bond of morphinone and codeinone, yielding hydromorphone and hydrocodone respectively. This saturation reaction is assisted by a FMN cofactor and the enzyme is a member of the α/β-barrel flavoprotein family. The sequence of the enzyme has been obtained from bacteria Pseudomonas putida M10 and has been successfully expressed in yeast and other bacterial species. The enzyme is reported to harbor high sequence and structural similarity to the Old Yellow Enzyme, a large group of flavin-dependent redox biocatalysts of yeast species, and an oestrogen-binding protein of Candida albicans. The enzyme has demonstrated value in biosynthesis of semi-opiate drugs in microorganisms, expanding the chemical diversity of BIA biosynthesis.








