
Corn smut is a plant disease caused by the pathogenic fungus Ustilago maydis that causes smut on maize and teosinte. The fungus forms galls on all above-ground parts of corn species. It is edible, and is known in Mexico as the delicacy huitlacoche; which is eaten, usually as a filling, in quesadillas and other tortilla-based foods, and in soups.

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi and serve primarily to transport iron across cell membranes, although a widening range of siderophore functions is now being appreciated. Siderophores are among the strongest soluble Fe3+ binding agents known.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.
A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometalations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometalation.
D-amino-acid dehydrogenase is a bacterial enzyme that catalyses the oxidation of D-amino acids into their corresponding oxoacids. It contains both flavin and nonheme iron as cofactors. The enzyme has a very broad specificity and can act on most D-amino acids.

Dimorphic fungi are fungi that can exist in the form of both mold and yeast. This is usually brought about by change in temperature and the fungi are also described as thermally dimorphic fungi. An example is Talaromyces marneffei, a human pathogen that grows as a mold at room temperature, and as a yeast at human body temperature.

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.
4-Hydroxy-3-methylbut-2-enyl diphosphate reductase (EC 1.17.1.2, isopentenyl-diphosphate:NADP+ oxidoreductase, LytB, (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate reductase, HMBPP reductase, IspH, LytB/IspH) is an enzyme in the non-mevalonate pathway. It acts upon (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (or "HMB-PP").

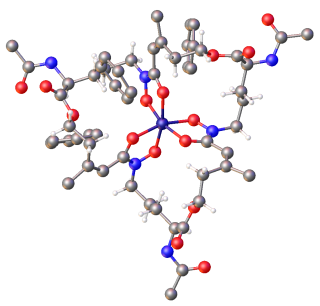
Ferrichrome is a cyclic hexa-peptide that forms a complex with iron atoms. It is a siderophore composed of three glycine and three modified ornithine residues with hydroxamate groups [-N(OH)C(=O)C-]. The 6 oxygen atoms from the three hydroxamate groups bind Fe(III) in near perfect octahedral coordination.

The Ustilaginaceae are a family of smut fungi in the order Ustilaginomycetes. Collectively, the family contains 17 genera and 607 species.
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.

Yersiniabactin (Ybt) is a siderophore found in the pathogenic bacteria Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica, as well as several strains of enterobacteria including enteropathogenic Escherichia coli and Salmonella enterica. Siderophores, compounds of low molecular mass with high affinities for ferric iron, are important virulence factors in pathogenic bacteria. Iron—an essential element for life used for such cellular processes as respiration and DNA replication—is extensively chelated by host proteins like lactoferrin and ferritin; thus, the pathogen produces molecules with an even higher affinity for Fe3+ than these proteins in order to acquire sufficient iron for growth. As a part of such an iron-uptake system, yersiniabactin plays an important role in pathogenicity of Y. pestis, Y. pseudotuberculosis, and Y. entercolitica.
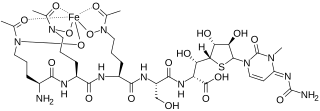
Albomycins are a group of naturally occurring antibiotics belonging to the class of sideromycins, which are "compounds composed of iron carriers called siderophores linked to antibiotic moieties". They are particularly effective against Gram-negative bacteria of the family Enterobacteriaceae and few Gram-positive bacteria such s Streptococcus pneumoniae, Bacillus subtilis and Staphylococcus aureus. In 2000 a group of scientists from SmithKline Beecham Pharmaceuticals, UK reported that the antibiotic part of albomycin in vitro can inhibit seryl t-RNA synthetase from both eukaryotic and prokaryotic representatives.
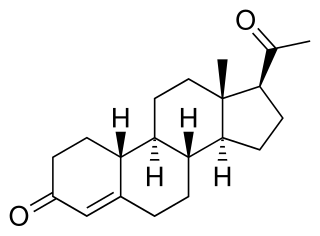
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.
N2-citryl-N6-acetyl-N6-hydroxylysine synthase (EC 6.3.2.38, N(alpha)-citryl-N(epsilon)-acetyl-N(epsilon)-hydroxylysine synthase, iucA (gene)) is an enzyme with systematic name citrate:N6-acetyl-N6-hydroxy-L-lysine ligase (ADP-forming). This enzyme catalyses the following chemical reaction

Bacillibactin is a catechol-based siderophore secreted by members of the genus Bacillus, including Bacillus anthracis and Bacillus subtilis. It is involved in the chelation of ferric iron (Fe3+) from the surrounding environment and is subsequently transferred into the bacterial cytoplasm via the use of ABC transporters.

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.
The Mukaiyama hydration is an organic reaction involving formal addition of an equivalent of water across an olefin by the action of catalytic bis(acetylacetonato)cobalt(II) complex, phenylsilane and atmospheric oxygen to produce an alcohol with Markovnikov selectivity.
Complex lasso proteins are proteins in which a covalent loop is pierced by another piece of the backbone. Subclass of complex lasso proteins are Lasso peptides in which the loop is formed by post-translational amide bridge.













