Related Research Articles

Post-transplant lymphoproliferative disorder (PTLD) is the name given to a B cell proliferation due to therapeutic immunosuppression after organ transplantation. These patients may develop infectious mononucleosis-like lesions or polyclonal polymorphic B-cell hyperplasia. Some of these B cells may undergo mutations which will render them malignant, giving rise to a lymphoma.

Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.
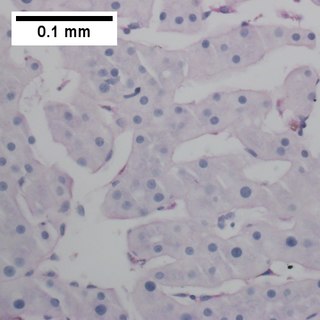
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.
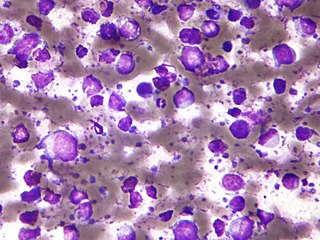
The B-cell lymphomas are types of lymphoma affecting B cells. Lymphomas are "blood cancers" in the lymph nodes. They develop more frequently in older adults and in immunocompromised individuals.

Intravascular lymphomas (IVL) are rare cancers in which malignant lymphocytes proliferate and accumulate within blood vessels. Almost all other types of lymphoma involve the proliferation and accumulation of malignant lymphocytes in lymph nodes, other parts of the lymphatic system, and various non-lymphatic organs but not in blood vessels.
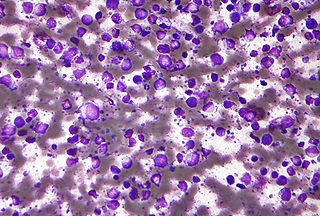
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.
Lymphomatoid granulomatosis (LYG or LG) is a very rare lymphoproliferative disorder first characterized in 1972. Lymphomatoid means lymphoma-like and granulomatosis denotes the microscopic characteristic of the presence of granulomas with polymorphic lymphoid infiltrates and focal necrosis within it.
Richter's transformation (RT), also known as Richter's syndrome, is the conversion of chronic lymphocytic leukemia (CLL) or its variant, small lymphocytic lymphoma (SLL), into a new and more aggressively malignant disease. CLL is the circulation of malignant B lymphocytes with or without the infiltration of these cells into lymphatic or other tissues while SLL is the infiltration of these malignant B lymphocytes into lymphatic and/or other tissues with little or no circulation of these cells in the blood. CLL along with its SLL variant are grouped together in the term CLL/SLL.
There are several forms of Epstein–Barr virus (EBV) infection. These include asymptomatic infections, the primary infection, infectious mononucleosis, and the progression of asymptomatic or primary infections to: 1) any one of various Epstein–Barr virus-associated lymphoproliferative diseases such as chronic active EBV infection, EBV+ hemophagocytic lymphohistiocytosis, Burkitt's lymphoma, and Epstein–Barr virus positive diffuse large B-cell lymphoma, not otherwise specified); 2) non-lymphoid cancers such as Epstein–Barr virus associated gastric cancer, soft tissue sarcomas, leiomyosarcoma, and nasopharyngeal cancers; and 3) Epstein–Barr virus-associated non-lymphoproliferative diseases such as some cases of the immune disorders of multiple sclerosis and systemic lupus erythematosis and the childhood disorders of Alice in Wonderland Syndrome and acute cerebellar ataxia.

Extranodal NK/T-cell lymphoma, nasal type (ENKTCL-NT) is a rare type of lymphoma that commonly involves midline areas of the nasal cavity, oral cavity, and/or pharynx At these sites, the disease often takes the form of massive, necrotic, and extremely disfiguring lesions. However, ENKTCL-NT can also involve the eye, larynx, lung, gastrointestinal tract, skin, and various other tissues. ENKTCL-NT mainly affects adults; it is relatively common in Asia and to lesser extents Mexico, Central America, and South America but is rare in Europe and North America. In Korea, ENKTCL-NT often involves the skin and is reported to be the most common form of cutaneous lymphoma after mycosis fungoides.

Plasmablastic lymphoma (PBL) is a type of large B-cell lymphoma recognized by the World Health Organization (WHO) in 2017 as belonging to a subgroup of lymphomas termed lymphoid neoplasms with plasmablastic differentiation. The other lymphoid neoplasms within this subgroup are: plasmablastic plasma cell lymphoma ; primary effusion lymphoma that is Kaposi's sarcoma-associated herpesvirus positive or Kaposi's sarcoma-associated Herpesvirus negative; anaplastic lymphoma kinase-positive large B-cell lymphoma; and human herpesvirus 8-positive diffuse large B-cell lymphoma, not otherwise specified. All of these lymphomas are malignancies of plasmablasts, i.e. B-cells that have differentiated into plasmablasts but because of their malignant nature: fail to differentiate further into mature plasma cells; proliferate excessively; and accumulate in and injure various tissues and organs.
Epstein–Barr virus positive diffuse large B-cell lymphoma, not otherwise specified is a form of diffuse large B-cell lymphomas (DLBCL) accounting for around 10-15% of DLBCL cases. DLBCL are lymphomas in which B-cell lymphocytes proliferate excessively, invade multiple tissues, and often causes life-threatening tissue damage. EBV+ DLBCL is distinguished from DLBCL in that virtually all the large B cells in the tissue, infiltrates of the Epstien-Barr virus (EBV) express EBV genes characteristic of the virus's latency III or II phase. EBV is a ubiquitous virus, infecting around 95% of the world population.
Epstein–Barr virus–associated lymphoproliferative diseases are a group of disorders in which one or more types of lymphoid cells, i.e. B cells, T cells, NK cells, and histiocytic-dendritic cells, are infected with the Epstein–Barr virus (EBV). This causes the infected cells to divide excessively, and is associated with the development of various non-cancerous, pre-cancerous, and cancerous lymphoproliferative disorders (LPDs). These LPDs include the well-known disorder occurring during the initial infection with the EBV, infectious mononucleosis, and the large number of subsequent disorders that may occur thereafter. The virus is usually involved in the development and/or progression of these LPDs although in some cases it may be an "innocent" bystander, i.e. present in, but not contributing to, the disease.

Mosquito bite allergies, also termed hypersensitivity to mosquito bites, are excessive reactions of varying severity to mosquito bites. They are allergic hypersensitivity reactions caused by the non-toxic allergenic proteins contained in the saliva injected by a female mosquito at the time it takes its blood meal, and are not caused by any toxin or pathogen. By general agreement, mosquito bite allergies do not include the ordinary wheal and flare responses to these bites although these reactions are also allergic in nature. Ordinary mosquito bite allergies are nonetheless detailed here because they are the best understood reactions to mosquito bites and provide a basis for describing what is understood about them.
Natural killer cell enteropathy, also termed NK cell enteropathy (NKCE), and a closely related disorder, lymphomatoid gastropathy (LG), are non-malignant diseases in which one type of lymphocyte, the natural killer cell, proliferates excessively in the gastrointestinal tract. This proliferation causes red, sore-like spots, raised lesions, erosions, and ulcers in the mucosal layer surrounding the GI tract lumen. Both disorders cause either no or only vague symptoms of GI tract disturbances such as nausea, vomiting, and bleeding.
T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is a malignancy of B cells. B-cells are lymphocytes that normally function in the humoral immunity component of the adaptive immune system by secreting antibodies that, for example, bind to and neutralize invasive pathogens. Among the various forms of B-cell lymphomas, THRLBCL is a rarely occurring subtype of the diffuse large B-cell lymphomas (DLBCL). DLBCL are a large group of lymphomas that account for ~25% of all non-Hodgkin lymphomas worldwide. THRLBCL is distinguished from the other DLBCL subtypes by the predominance of non-malignant T-cell lymphocytes and histiocytes over malignant B-cells in its tumors and tissue infiltrates.
Primary testicular diffuse large B-cell lymphoma (PT-DLBCL), also termed testicular diffuse large B-cell lymphoma and diffuse large B-cell lymphoma of the testes, is a variant of the diffuse large B-cell lymphomas (DLBCL). DLBCL are a large and diverse group of B-cell malignancies with the great majority (-85%) being typed as diffuse large B-cell lymphoma, not otherwise specified. PT-DLBCL is a variant of DLBCL, NOS that involves one or, in uncommon cases, both testicles. Other variants and subtypes of DLBCL may involve the testes by spreading to them from their primary sites of origin in other tissues. PT-DLBCL differs from these other DLBCL in that it begins in the testes and then may spread to other sites.
Diffuse large B-cell lymphoma associated with chronic inflammation (DLBCL-CI) is a subtype of the Diffuse large B-cell lymphomas and a rare form of the Epstein–Barr virus-associated lymphoproliferative diseases, i.e. conditions in which lymphocytes infected with the Epstein-Barr virus (EBV) proliferate excessively in one or more tissues. EBV infects ~95% of the world's population to cause no symptoms, minor non-specific symptoms, or infectious mononucleosis. The virus then enters a latency phase in which the infected individual becomes a lifetime asymptomatic carrier of the virus. Some weeks, months, years, or decades thereafter, a very small fraction of these carriers, particularly those with an immunodeficiency, develop any one of various EBV-associated benign or malignant diseases.

Lymphoid neoplasms with plasmablastic differentiation were classified by the World Health Organization, 2017 as a sub-grouping of several distinct but rare lymphomas in which the malignant cells are B-cell lymphocytes that have become plasmablasts, i.e. immature plasma cells. Normally, B-cells take up foreign antigens, move to the germinal centers of secondary lymphoid organs such the spleen and lymph nodes, and at these sites are stimulated by T-cell lymphocytes to differentiate into plasmablasts and thereafter mature plasma cells. Plasmablasts, and to a greater extent, plasma cells make and secrete antibodies that bind the antigens to which their predecessor B-cells were previously exposed. Antibodies function, in part, to neutralize harmful bacteria and viruses by binding antigens that are exposed on their surfaces. Due to their malignant nature, however, the plasmablasts in lymphoid neoplasms with plasmablastic differentiation do not mature into plasma cells or form antibodies but rather uncontrollably proliferate in and damage various tissues and organs. The individual lymphomas in this sub-group of malignancies have heterogeneous clinical, morphological, and gene findings that often overlap with other members of the sub-group. In consequence, correctly diagnosing these lymphomas has been challenging. Nonetheless, it is particularly important to diagnose them correctly because they often have very different prognoses and treatments. The lymphoid neoplasms with plasmacytic differentiation are:
References
- 1 2 3 4 Grimm KE, O'Malley DP (2019). "Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues". Annals of Diagnostic Pathology. 38: 6–10. doi:10.1016/j.anndiagpath.2018.09.014. PMID 30380402. S2CID 53196244.
- 1 2 3 4 5 6 7 8 9 10 11 12 Dojcinov SD, Fend F, Quintanilla-Martinez L (March 2018). "EBV-Positive Lymphoproliferations of B- T- and NK-Cell Derivation in Non-Immunocompromised Hosts". Pathogens (Basel, Switzerland). 7 (1): 28. doi: 10.3390/pathogens7010028 . PMC 5874754 . PMID 29518976.
- 1 2 3 4 5 6 7 8 9 Boyer DF, McKelvie PA, de Leval L, Edlefsen KL, Ko YH, Aberman ZA, Kovach AE, Masih A, Nishino HT, Weiss LM, Meeker AK, Nardi V, Palisoc M, Shao L, Pittaluga S, Ferry JA, Harris NL, Sohani AR (March 2017). "Fibrin-associated EBV-positive Large B-Cell Lymphoma: An Indolent Neoplasm With Features Distinct From Diffuse Large B-Cell Lymphoma Associated With Chronic Inflammation". The American Journal of Surgical Pathology. 41 (3): 299–312. doi:10.1097/PAS.0000000000000775. PMID 28195879. S2CID 3521190.
- 1 2 Korkolopoulou P, Vassilakopoulos T, Milionis V, Ioannou M (July 2016). "Recent Advances in Aggressive Large B-cell Lymphomas: A Comprehensive Review". Advances in Anatomic Pathology. 23 (4): 202–43. doi:10.1097/PAP.0000000000000117. PMID 27271843. S2CID 205915174.
- 1 2 Rezk SA, Zhao X, Weiss LM (June 2018). "Epstein—Barr virus-associated lymphoid proliferations, a 2018 update". Human Pathology. 79: 18–41. doi:10.1016/j.humpath.2018.05.020. PMID 29885408. S2CID 47010934.
- 1 2 3 4 5 6 7 8 9 10 Zanelli M, Zizzo M, Montanaro M, Gomes V, Martino G, De Marco L, Fraternali Orcioni G, Martelli MP, Ascani S (September 2019). "Fibrin-associated large B-cell lymphoma: first case report within a cerebral artery aneurysm and literature review". BMC Cancer. 19 (1): 916. doi: 10.1186/s12885-019-6123-1 . PMC 6743119 . PMID 31519155.
- 1 2 Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ (November 2019). "Diffuse large B-cell lymphoma variants: an update". Pathology. 52 (1): 53–67. doi:10.1016/j.pathol.2019.08.013. PMID 31735345. S2CID 208142227.
- 1 2 3 4 King RL, Goodlad JR, Calaminici M, Dotlic S, Montes-Moreno S, Oschlies I, Ponzoni M, Traverse-Glehen A, Ott G, Ferry JA (December 2019). "Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis". Virchows Archiv. 476 (5): 647–665. doi:10.1007/s00428-019-02698-3. PMID 31863183. S2CID 209429124.
- ↑ Boroumand N, Ly TL, Sonstein J, Medeiros LJ (July 2012). "Microscopic diffuse large B-cell lymphoma (DLBCL) occurring in pseudocysts: do these tumors belong to the category of DLBCL associated with chronic inflammation?". The American Journal of Surgical Pathology. 36 (7): 1074–80. doi:10.1097/PAS.0b013e3182515fb5. PMID 22472958. S2CID 31478084.