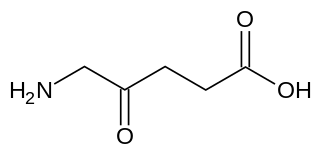
δ-Aminolevulinic acid, an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in plants.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Glutamate-1-semialdehyde is a molecule formed from by the reduction of tRNA bound glutamate, catalyzed by glutamyl-tRNA reductase. It is isomerized by glutamate-1-semialdehyde 2,1-aminomutase to give aminolevulinic acid in the biosynthesis of porphyrins, including heme and chlorophyll.
In enzymology, a succinylglutamate-semialdehyde dehydrogenase (EC 1.2.1.71) is an enzyme that catalyzes the chemical reaction
In enzymology, a dihydrokaempferol 4-reductase (EC 1.1.1.219) is an enzyme that catalyzes the chemical reaction

Sepiapterin reductase is an enzyme that in humans is encoded by the SPR gene.

In enzymology, an UDP-N-acetylmuramate dehydrogenase (EC 1.3.1.98) is an enzyme that catalyzes the chemical reaction

In enzymology, divinyl chlorophyllide a 8-vinyl-reductase (EC 1.3.1.75) is an enzyme that catalyzes the chemical reaction
In enzymology, a precorrin-6A reductase (EC 1.3.1.54) is an enzyme that catalyzes the chemical reaction
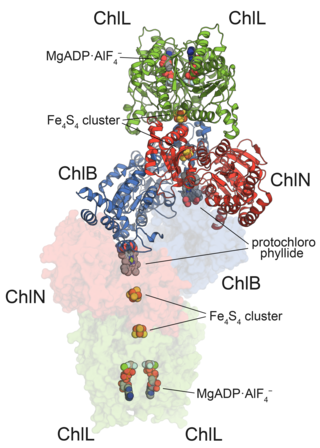
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide a. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.

In enzymology, an aspartate-semialdehyde dehydrogenase is an enzyme that is very important in the biosynthesis of amino acids in prokaryotes, fungi, and some higher plants. It forms an early branch point in the metabolic pathway forming lysine, methionine, leucine and isoleucine from aspartate. This pathway also produces diaminopimelate which plays an essential role in bacterial cell wall formation. There is particular interest in ASADH as disabling this enzyme proves fatal to the organism giving rise to the possibility of a new class of antibiotics, fungicides, and herbicides aimed at inhibiting it.
In enzymology, a glutamate-5-semialdehyde dehydrogenase (EC 1.2.1.41) is an enzyme that catalyzes the chemical reaction

In enzymology, a L-aminoadipate-semialdehyde dehydrogenase (EC 1.2.1.31) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetyl-gamma-glutamyl-phosphate reductase (EC 1.2.1.38) is an enzyme that catalyzes the chemical reaction

In enzymology, a saccharopine dehydrogenase (NADP+, L-glutamate-forming) (EC 1.5.1.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a saccharopine dehydrogenase (NADP+, L-lysine-forming) (EC 1.5.1.8) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutamate—tRNA ligase is an enzyme that catalyzes the chemical reaction
Succinate-semialdehyde dehydrogenase (NADP+) (EC 1.2.1.79, succinic semialdehyde dehydrogenase (NADP+), succinyl semialdehyde dehydrogenase (NADP+), succinate semialdehyde:NADP+ oxidoreductase, NADP-dependent succinate-semialdehyde dehydrogenase, GabD) is an enzyme with systematic name succinate-semialdehyde:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
Geranylgeranyl diphosphate reductase (EC 1.3.1.83, geranylgeranyl reductase, CHL P) is an enzyme with systematic name geranylgeranyl-diphosphate:NADP+ oxidoreductase. This enzyme catalises the following chemical reaction

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.










