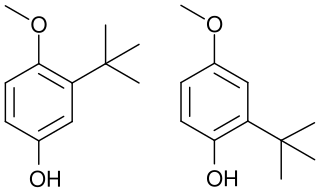
Butylated hydroxyanisole (BHA) is a synthetic, waxy, solid petrochemical. Its antioxidant properties have caused it to be widely used as a preservative in food, food packaging, animal feed, cosmetics, pharmaceuticals, rubber, and petroleum products. BHA has been used in food since around 1947.

Malignancy is the tendency of a medical condition to become progressively worse; the term is most familiar as a characterization of cancer.
Spontaneous remission, also called spontaneous healing or spontaneous regression, is an unexpected improvement or cure from a disease that usually progresses. These terms are commonly used for unexpected transient or final improvements in cancer. Spontaneous remissions concern cancers of the haematopoietic system, while spontaneous regressions concern palpable tumors; however, both terms are often used interchangeably.

Johannes Andreas Grib Fibiger was a Danish physician and professor of anatomical pathology at the University of Copenhagen. He was the recipient of the 1926 Nobel Prize in Physiology or Medicine "for his discovery of the Spiroptera carcinoma". He demonstrated that the roundworm which he called Spiroptera carcinoma could cause stomach cancer in rats and mice. His experimental results were later proven to be a case of mistaken conclusion. Erling Norrby, who had served as the Permanent Secretary of the Royal Swedish Academy of Sciences and Professor and Chairman of Virology at the Karolinska Institute, declared Fibiger's Nobel Prize as "one of the biggest blunders made by the Karolinska Institute."

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites, more commonly known as BPDE, react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.
Malignant transformation is the process by which cells acquire the properties of cancer. This may occur as a primary process in normal tissue, or secondarily as malignant degeneration of a previously existing benign tumor.
Since the first award in 1901, conferment of the Nobel Prize has engendered criticism and controversy. After his death in 1896, the will of Swedish industrialist Alfred Nobel established that an annual prize be awarded for service to humanity in the fields of physics, chemistry, physiology or medicine, literature, and peace. Similarly, the Sveriges Riksbank Prize in Economic Sciences in Memory of Alfred Nobel is awarded along with the Nobel Prizes.

Carcinoembryonic antigen (CEA) describes a set of highly-related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. Consequently, CEA is usually present at very low levels in the blood of healthy adults. However, the serum levels are raised in some types of cancer, which means that it can be used as a tumor marker in clinical tests. Serum levels can also be elevated in heavy smokers.
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses. Oncolytic viruses also have the ability to affect the tumor micro-environment in multiple ways.

A precancerous condition is a condition, tumor or lesion involving abnormal cells which are associated with an increased risk of developing into cancer. Clinically, precancerous conditions encompass a variety of abnormal tissues with an increased risk of developing into cancer. Some of the most common precancerous conditions include certain colon polyps, which can progress into colon cancer, monoclonal gammopathy of undetermined significance, which can progress into multiple myeloma or myelodysplastic syndrome. and cervical dysplasia, which can progress into cervical cancer. Bronchial premalignant lesions can progress to squamous cell carcinoma of the lung.

Francis Peyton Rous was an American pathologist at the Rockefeller University known for his works in oncoviruses, blood transfusion and physiology of digestion. A medical graduate from the Johns Hopkins University, he was discouraged from becoming a practicing physician due to severe tuberculosis. After three years of working as an instructor of pathology at the University of Michigan, he became dedicated researcher at the Rockefeller Institute for Medical Research for the rest of his career.

Gongylonema is a genus of thread-like nematode that was described by Molin in 1857. It is the only currently valid genus in the family Gongylonematidae, though the mysterious Spiruroides – usually placed in the Subuluridae, which are not closely related to Gongylonema among the Spiruria – might actually belong here. They are parasites of birds and mammals, transmitted by insects. Some 38 species are known, about 12 of which have been recorded in Europe.

miR-218 microRNA precursor is a small non-coding RNA that regulates gene expression by antisense binding.
Alopecia neoplastica may present as a scarring alopecia, appearing anywhere on the scalp, and it has been described with cutaneous metastasis from breast, gastric, lung, renal and pancreatic carcinomas.
miR-31 has been characterised as a tumour suppressor miRNA, with its levels varying in breast cancer cells according to the metastatic state of the tumour. From its typical abundance in healthy tissue is a moderate decrease in non-metastatic breast cancer cell lines, and levels are almost completely absent in mouse and human metastatic breast cancer cell lines. Mir-31-5p has also been observed upregulated in Zinc Deficient rats compared to normal in ESCC and in other types of cancers when using this animal model. There has also been observed a strong encapsulation of tumour cells expressing miR-31, as well as a reduced cell survival rate. miR-31's antimetastatic effects therefore make it a potential therapeutic target for breast cancer. However, these two papers were formally retracted by the authors in 2015.

Yamagiwa Katsusaburō was a Japanese pathologist who carried out pioneering work into the causes of cancer, and was the first to demonstrate chemical carcinogenesis. He was a 7-time Nobel Prize nominee.
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation. Macrophages are known to originate from bone marrow-derived blood monocytes or yolk sac progenitors, but the exact origin of TAMs in human tumors remains to be elucidated. The composition of monocyte-derived macrophages and tissue-resident macrophages in the tumor microenvironment depends on the tumor type, stage, size, and location, thus it has been proposed that TAM identity and heterogeneity is the outcome of interactions between tumor-derived, tissue-specific, and developmental signals.

Louis Westenra Sambon was an Italian-English physician who played important roles in understanding the causes (etiology) of diseases. He described many pathogenic protozoans, insects, and helminths including the name Schistosoma mansoni for a blood fluke. He was an authority on the classification of parasitic tongue worms called Pentastomida (Linguatulida), and one of the genus Sambonia is named after him.
Carcinogenic parasites are parasitic organisms that depend on other organisms for their survival, and cause cancer in such hosts. Three species of flukes (trematodes) are medically-proven carcinogenic parasites, namely the urinary blood fluke, the Southeast Asian liver fluke and the Chinese liver fluke. S. haematobium is prevalent in Africa and the Middle East, and is the leading cause of bladder cancer. O. viverrini and C. sinensis are both found in eastern and southeastern Asia, and are responsible for cholangiocarcinoma. The International Agency for Research on Cancer declared them in 2009 as a Group 1 biological carcinogens in humans.











