Antiviral drugs are a class of medication used specifically for treating viral infections rather than bacterial ones. Most antivirals are used for specific viral infections, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit their development.
Antigenic drift is a mechanism for variation in viruses that involves the accumulation of mutations within the genes that code for antibody-binding sites. This results in a new strain of virus particles which cannot be inhibited as effectively by the antibodies that were originally targeted against previous strains, making it easier for the virus to spread throughout a partially immune population. Antigenic drift occurs in both influenza A and influenza B viruses.
Live attenuated influenza vaccine (LAIV) is a type of influenza vaccine in the form of a nasal spray that is recommended for the prevention of influenza. In June 2016 the CDC stopped recommending the use of LAIV as its effectiveness has appeared to have decreased between 2013 and 2016, but this recommendation was reversed in February 2018 for the 2018-2019 influenza season.

The 1968 flu pandemic was a category 2 flu pandemic whose outbreak in 1968 and 1969 killed an estimated one million people worldwide. It was caused by an H3N2 strain of the influenza A virus, descended from H2N2 through antigenic shift, a genetic process in which genes from multiple subtypes reassorted to form a new virus. Because it originated in Hong Kong, the pandemic is also referred to as Hong Kong flu.
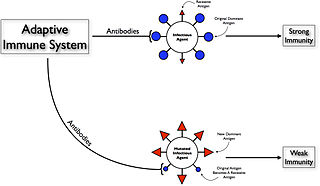
Original antigenic sin, also known as the Hoskins effect, refers to the propensity of the body's immune system to preferentially utilize immunological memory based on a previous infection when a second slightly different version of that foreign entity is encountered. This leaves the immune system "trapped" by the first response it has made to each antigen, and unable to mount potentially more effective responses during subsequent infections. The phenomenon of original antigenic sin has been described in relation to influenza virus, dengue fever, human immunodeficiency virus (HIV), and to several other viruses.

Influenza (H1N1) virus is the subtype of influenza A virus that was the most common cause of human influenza (flu) in 2009, and is associated with the 1918 outbreak known as the Spanish flu.

Canine influenza is influenza occurring in canine animals. Canine influenza is caused by varieties of influenzavirus A, such as equine influenza virus H3N8, which in 2004 was discovered to cause disease in dogs. Because of the lack of previous exposure to this virus, dogs have no natural immunity to it. Therefore, the disease is rapidly transmitted between individual dogs. Canine influenza may be endemic in some regional dog populations of the United States. It is a disease with a high morbidity but a low incidence of death.
Lymphocytopenia is the condition of having an abnormally low level of lymphocytes in the blood. Lymphocytes are a white blood cell with important functions in the immune system. The opposite is lymphocytosis, which refers to an excessive level of lymphocytes.

Transmission and infection of H5N1 from infected avian sources to humans has been a concern since the first documented case of human infection in 1997, due to the global spread of H5N1 that constitutes a pandemic threat.

Influenza C virus is the species in the genus Influenzavirus C in the virus family Orthomyxoviridae, which like other influenza viruses, causes influenza.

Treatments for influenza include a range of medications and therapies that are used in response to disease influenza. Treatments may either directly target the influenza virus itself; or instead they may just offer relief to symptoms of the disease, while the body's own immune system works to recover from infection.

Influenza research involves investigating molecular virology, pathogenesis, host immune responses, genomics, and epidemiology regarding influenza. The main goal of research is to develop influenza countermeasures such as vaccines, therapies and diagnostic tools.

Umifenovir is an antiviral treatment for influenza infection used in Russia and China. The drug is manufactured by Pharmstandard. Although some Russian studies have shown it to be effective, it is not approved for use in Western countries. Chemically, umifenovir features an indole core, functionalized at all but one positions with different substituents. The drug inhibits viral entry into target cells and also stimulates the immune response.

Human mortality from H5N1 or the human fatality ratio from H5N1 or the case-fatality rate of H5N1 refer to the ratio of the number of confirmed human deaths resulting from confirmed cases of transmission and infection of H5N1 to the number of those confirmed cases. For example, if there are 100 confirmed cases of humans infected with H5N1 and 10 die, then there is a 10% human fatality ratio. H5N1 flu is a concern due to the global spread of H5N1 that constitutes a pandemic threat. The majority of H5N1 flu cases have been reported in southeast and east Asia. The case-fatality rate is central to pandemic planning. While estimates of case-fatality (CF) rates for past influenza pandemics have ranged from about 0.1% to 2.5% ; the official World Health Organization estimate for the current outbreak of H5N1 avian influenza to date is around 60%. While the real H5N1 CF rate could be lower ; it is unlikely that, if it becomes a pandemic, it will go to the 0.1–0.4% level currently embraced by many pandemic plans.

H2N3 is a subtype of the influenza A virus. Its name derives from the forms of the two kinds of proteins on the surface of its coat, hemagglutinin (H) and neuraminidase (N). H2N3 viruses can infect birds and mammals.
An inactivated vaccine is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then killed. In contrast, live vaccines use pathogens that are still alive. Pathogens for inactivated vaccines are grown under controlled conditions and are killed as a means to reduce infectivity (virulence) and thus prevent infection from the vaccine. The virus is killed using a method such as heat or formaldehyde.
FI6 is an antibody that targets a protein found on the surface of all influenza A viruses called hemagglutinin. FI6 is the only known antibody found to bind all 16 subtypes of the influenza A virus hemagglutinin and is hoped to be useful for a universal influenza virus therapy.

H7N9 is a bird flu strain of the species Influenza virus A. Avian influenza A H7 viruses normally circulate amongst avian populations with some variants known to occasionally infect humans. An H7N9 virus was first reported to have infected humans in March 2013, in China. Cases continued to be reported throughout April and then dropped to only a few cases during the summer months. At the closing of the year, 144 cases had been reported of which 46 had died. It is known that influenza tends to strike during the winter months, and the second wave, which began in October, was fanned by a surge in poultry production timed for Chinese New Year feasts that began at the end of January. January 2014 brought a spike in reports of illness with 96 confirmed reports of disease and 19 deaths. As of April 11, 2014, the outbreak's overall total was 419, including 7 in Hong Kong, and the unofficial number of deaths was 127.
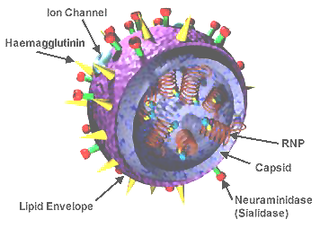
Influenza D virus is a species in the virus genus Influenzavirus D in the family Orthomyxoviridae, that causes influenza.









