Current status of H5N1 candidate vaccines
Candidate vaccines were developed in the United States and the United Kingdom during 2003 for protection against the strain that was isolated from humans in Hong Kong in February 2003 but the 2003 strain died out in 2004 making the vaccine of little use. In April 2004, WHO made an H5N1 prototype seed strain available to manufacturers. In August 2006, WHO changed the prototype strains and now offers three new prototype strains which represent three of the six subclades of the clade 2 virus which have been responsible for many of the human cases that have occurred since 2005.
The National Institute of Allergy and Infectious Diseases (NIAID) awarded H5N1 vaccine contracts to Aventis Pasteur (now Sanofi Pasteur) of Swiftwater, Pennsylvania, and to Chiron Corporation of Emeryville, California. Each manufacturer is using established techniques in which the virus is grown in eggs and then inactivated and further purified before being formulated into vaccines.
"A universal influenza vaccine could provide protection against all types of influenza and would eliminate the need to develop individual vaccines to specific H and N virus types. Such a vaccine would not need to be reengineered each year and could protect against an emergent pandemic strain. Developing a universal vaccine requires that researchers identify conserved regions of the influenza virus that do not exhibit antigenic variability by strain or over time. A universal vaccine, ACAM-FLU-A, is being developed by the British company Acambis and is being researched by others as well. Acambis (meanwhile also acquired by Sanofi Pasteur) announced in early August 2005 that it has had successful results in animal testing. The vaccine focuses on the M2 viral protein, which does not change, rather than the surface hemagglutinin and neuraminidase proteins targeted by traditional flu vaccines. The universal vaccine is made through bacterial fermentation technology, which would greatly speed up the rate of production over that possible with culture in chicken eggs, plus the vaccine could be produced constantly, since its formulation would not change. Still, such a vaccine is years away from full testing, approval, and use." [2] As of July 2007, phase I clinical trials on humans are underway in which a vaccine that focuses on the M2 viral protein "is being administered to a small group of healthy people in order to verify the safety of the product and to provide an initial insight into the vaccine’s effect on the human immune system." [3] (See also Universal flu vaccines) The current development state of ACAM-FLU-A is unclear. [4]
In June 2006, the National Institutes of Health (NIH) began enrolling participants in a Phase 1 H5N1 study of an intranasal influenza vaccine candidate based on MedImmune's live, attenuated vaccine technology. [5]
Oct 2010 Inovio starts a phase I clinical trial of its H5N1 vaccine (VGX-3400X). [6]
Oct 2012 Novavax, Inc. pandemic influenza vaccine Phase I trials meet primary objectives. [7]
Approved human H5N1 vaccines
On April 17, 2007 the US FDA approved "Influenza Virus Vaccine, H5N1" by manufacturer Sanofi Pasteur Inc for manufacture at its Swiftwater, PA facility. [8]
In March 2006, Hungarian Prime Minister Ferenc Gyurcsány reported that Omninvest developed a vaccine to protect humans against the H5N1 influenza strain. The vaccine was approved by the country's national pharmaceutical institute for commercial production. [9]
Results of trials
Early results from H5N1 clinical trials showed poor immunogenicity compared to the 15-mcg dose that induces immunity in a seasonal flu vaccine. Trials in 2006 and 2007 using two 30-mcg doses produced unacceptable results while a 2006 trial using two doses of 90 mcg each achieved acceptable levels of protection. Current flu vaccine manufacturing plants can not produce enough pandemic flu vaccine at this high dose level. [10]
"Adjuvanted vaccines appear to hold the greatest promise for solving the grave supply-demand imbalance in pandemic influenza vaccine development. They come with obstacles—immunologic, regulatory, and commercial—but they also have generated more excitement than any other type of vaccine thus far. [In August 2007], scientists working with a GlaxoSmithKline formula published a trial of a two-dose regimen of an inactivated split-virus vaccine adjuvanted with a proprietary oil-in-water emulsion; after the second injection, even the lowest dose of 3.8 mcg exceeded EU criteria for immune response (see Bibliography: Leroux-Roels 2007). And in September, Sanofi Pasteur reported in a press release that an inactivated vaccine adjuvanted with the company's own proprietary formula induced EU-accepted levels of protection after two doses of 1.9 mcg." [11]
The "GlaxoSmithKline-backed team that described an acceptable immune response after two adjuvanted 3.8-microgram (mcg) doses found that three fourths of their subjects were protected not only against the clade 1 Vietnam virus on which the vaccine was based, but against a drifted clade 2 virus from Indonesia as well [...] To achieve prepandemic vaccines, researchers would have to ascertain the right dose and dose interval, determine how long priming lasts, and solve the puzzle of measuring primed immunity. Further, regulatory authorities would have to determine the trial design that could deliver those answers, the public discussion that would be necessary for prepandemic vaccines to be accepted, and the safety data that would need to be gathered once the vaccines went into use". [12]
Individual studies
Revaccination - January 2006
Study completion: January 2006
The purpose of this study is to determine whether having received an H5 vaccine in the past primes the immune system to respond rapidly to another dose of H5 vaccine. Subjects who participate in this study will have participated in a previous vaccine study (involving the A/Hong/Kong/97 virus) during the fall of 1998 at the University of Rochester. [13]
A/H5N1 in adult - February 2006
Study start: April 2005; Study completion: February 2006
The purpose of this study is to determine the dose-related safety of flu vaccine in healthy adults. To determine the dose-related effectiveness of flu vaccine in healthy adults approximately 1 month following receipt of 2 doses of vaccine. To provide information for the selection of the best dose levels for further studies. [14]
H5 booster after two doses - June 2006
Study start: October 2005; Study completion: June 2006
The purpose of this study is to determine whether a third dose of vaccines containing A/Vietnam/1203/04 provides more immunity than two doses. Subjects who participate in this study, will have participated in DMID protocol 04-063 involving the A/Vietnam/1203/04. In this study, each subject will be asked to receive a third dose of the H5 vaccine at the same level administered in protocol 04-063. [15]
H5 in the elderly - August 2006
Study start: October 2005; Study completion: August 2006
This study is intended to examine the safety and dose-related immunogenicity of three dosage levels of the Influenza A/H5N1 vaccine, as compared to saline placebo, given intramuscularly to healthy elderly adults approximately 4 weeks apart. [16]
H5 in healthy adults - November 2006
Study start: March 2006; Expected completion: November 2006
This randomized, controlled, double-blinded, dose-ranging, Phase I-II study in 600 healthy adults, 18 to 49 years old, is designed to investigate the safety, reactogenicity, and dose-related immunogenicity of an investigational inactivated influenza A/H5N1 virus vaccine when given alone or combined with aluminum hydroxide. A secondary goal is to guide selection of vaccine dosage levels for expanded Phase II trials based on reactogenicity and immunogenicity profiles. This dose optimization will be applied to both younger and older subject populations in subsequent studies. Subjects who meet the entry criteria for the study will be enrolled at one of up to 5 study sites and will be randomized into 8 groups to receive two doses of influenza A/H5N1 vaccine containing 3.75, 7.5, 15, or 45 mcg of HA with or without aluminum hydroxide adjuvant by IM injection (N= 60 or 120/vaccine dose group). [17]
Bird flu - November 2006
Study start: March 2006; Study completion: November 2006
This study is designed to gather critical information on the safety, tolerability, and the immunogenicity (capability of inducing an immune response) of A/H5N1 virus vaccine in healthy adults. Up to 280 healthy adults, aged 18 to 64, will participate in the study. Each subject will participate for 7 months and will be randomly placed in one of several different study groups receiving a different dose of vaccine, vaccine plus adjuvant, or placebo. All subjects will receive two injections of their assigned study product, about 28 days apart, in their muscle tissue. Subjects will keep a journal of their temperature and any adverse effects between study visits. A small amount of blood will also be drawn before the first injection, 7 days after each injection, and 6 months after the second injection. [18]
Pandemic flu - January 2007
Study start: October 2005; Study completion: January 2007
This Australian study will test the safety and immunogenicity of an H5N1 pandemic influenza vaccine in healthy adults. [19]
Children - February 2007
Study start: January 2006; Study completion: February 2007
This is a randomized, double-blinded, placebo-controlled, staged, dose-ranging, Phase I/II study to evaluate the safety, reactogenicity, and immunogenicity of 2 doses of an IM inactivated influenza A/H5N1 vaccine in healthy children, aged 2 through 9 years. This study is designed to investigate the safety, tolerability, and dose-related immunogenicity of an investigational inactivated influenza A/H5N1 vaccine. A secondary goal is to identify an optimal dosage level of the vaccine that generates an acceptable immunogenic response, while maintaining an adequate safety profile. [20]

Influenza vaccines, also known as flu shots or flu jabs, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high quality research. Vaccinating children may protect those around them.
Live attenuated influenza vaccine (LAIV) is a type of influenza vaccine in the form of a nasal spray that is recommended for the prevention of influenza. In June 2016, the Centers for Disease Control and Prevention (CDC) stopped recommending the use of LAIV as its effectiveness had appeared to have decreased between 2013 and 2016, but this recommendation was reversed in February 2018, for the 2018-2019 influenza season.

Seasonal influenza vaccine brands include Fluzone/Fluzone Quadrivalent and Vaxigrip/VaxigripTetra, Influvac and Optaflu.

An influenza pandemic is an epidemic of an influenza virus that spreads across a large region and infects a large proportion of the population. There have been five in the last 140 years, with the 1918 flu pandemic being the most severe; this pandemic is estimated to have been responsible for the deaths of 50–100 million people. The most recent, the 2009 swine flu pandemic, resulted in under a million deaths and is considered relatively mild. These pandemics occur irregularly.

Transmission and infection of H5N1 from infected avian sources to humans has been a concern since the first documented case of human infection in 1997, due to the global spread of H5N1 that constitutes a pandemic threat.

Influenza research involves investigating molecular virology, pathogenesis, host immune responses, genomics, and epidemiology regarding influenza. The main goal of research is to develop influenza countermeasures such as vaccines, therapies and diagnostic tools.

Pandemrix is an influenza vaccine for influenza pandemics, such as the 2009 flu pandemic. The vaccine was developed by GlaxoSmithKline (GSK) and patented in September 2006.

The 2009 flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed vaccine was injected, while the live vaccine was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009.
AS03 is the trade name for a squalene-based immunologic adjuvant used in various vaccine products by GlaxoSmithKline (GSK). It is used, for example, in GSK's A/H1N1 pandemic flu vaccine Pandemrix. It is also in Arepannix and the Q-pan for H5N1 influenza.
Zoltan Vajo is a Hungarian/American scientist, best known for his contributions to the Human Genome Project, including cloning the COQ7 gene, characterizing the human CLK-1 timing protein cDNA and its potential effect on aging, and research on the molecular and genetic background of skeletal dysplasias and fibroblast growth factor receptor 3 disorders, including Achondroplasia, SADDAN, Thanatophoric dysplasia, Muenke coronal craniosynostosis and Crouzon syndrome as well as more recently on genetically engineered insulin analog molecules, including their structure, metabolic effects and cellular processing and the role of recombinant DNA technology in the treatment of diabetes.

A H5N1 vaccine is an influenza vaccine intended to provide immunization to influenza A virus subtype H5N1.
Cell-based vaccines are developed from mammalian cell lines rather than the more common method which uses the cells in embryonic chicken eggs to develop the antigens. The potential use of cell culture techniques in developing viral vaccines has been widely investigated in recent years as a complementary and alternative platform to the current egg-based strategies.
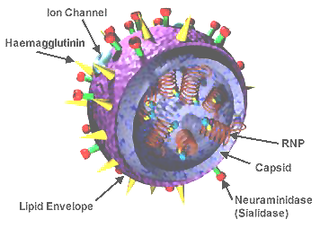
A universal flu vaccine is a flu vaccine that is effective against all influenza strains regardless of the virus sub type, antigenic drift or antigenic shift. Hence it should not require modification from year to year. As of 2019 there has been no approved universal flu vaccine for general use, but several have been in development.
Valneva SE is a French biotech company headquartered in Saint-Herblain, France, developing and commercializing vaccines for infectious diseases. It has manufacturing sites in Livingston Scotland, Solna Sweden and Vienna Austria, with other offices in France, Canada and the United States.
A Zika virus vaccine is designed to prevent the symptoms and complications of Zika virus infection in humans. As Zika virus infection of pregnant women may result in congenital defects in the newborn, the vaccine will attempt to protect against congenital Zika syndrome during the current or any future outbreak. As of April 2019, no vaccines have been approved for clinical use, however a number of vaccines are currently in clinical trials. The goal of a Zika virus vaccine is to produce specific antibodies against the Zika virus to prevent infection and severe disease. The challenges in developing a safe and effective vaccine include limiting side effects such as Guillain-Barré syndrome, a potential consequence of Zika virus infection. Additionally, as dengue virus is closely related to Zika virus, the vaccine needs to minimize the possibility of antibody-dependent enhancement of dengue virus infection.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus causing coronavirus disease 2019 (COVID‑19). Prior to the COVID‑19 pandemic, there was an established body of knowledge about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which enabled accelerated development of various vaccine technologies during early 2020. On 10 January 2020, the SARS-CoV-2 genetic sequence data was shared through GISAID, and by 19 March, the global pharmaceutical industry announced a major commitment to address COVID-19.

Medicago Inc. is a Canadian biotechnology company focused on the discovery, development, and commercialization of virus-like particles using plants as "bioreactors" to produce proteins as candidate vaccines and medications. By using live plant leaves as hosts in the discovery and manufacturing process, the Medicago "Proficia" technology has the goal to create a rapid, high-yield system for its product candidates.

VLA2001, also known as the Valneva COVID-19 vaccine, is a COVID-19 vaccine candidate developed by French biotechnology company Valneva SE in collaboration with Dynavax Technologies. It is an inactivated whole virus vaccine, grown in culture using the Vero cell line.

Sanofi–GSK COVID-19 vaccine also known as VAT00002 is a COVID-19 vaccine candidate developed by Sanofi Pasteur and GSK.








