In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air.

Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-red colored solid which melts into a red liquid at −163 °C (110 K). It is an extremely strong oxidant and decomposes into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% per day — its lifetime at room temperature is thus extremely short. Dioxygen difluoride reacts vigorously with nearly every chemical it encounters (including ordinary ice) leading to its onomatopoeic nickname FOOF (a play on its chemical structure and its explosive tendencies).

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.

Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour. The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol (F
3C)
2C(OH)
2, a geminal diol.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
Mercury(II) fluoride has the molecular formula HgF2 as a chemical compound of one atom of mercury with 2 atoms of fluorine.

Mercury(IV) fluoride, HgF4, is the first mercury compound to be reported with mercury in the +4 oxidation state. Mercury, like the other group 12 elements (cadmium and zinc), has an s2d10 electron configuration and generally only forms bonds involving its 6s orbital. This means that the highest oxidation state mercury normally attains is +2, and for this reason it is sometimes considered a post-transition metal instead of a transition metal. HgF4 was first reported from experiments in 2007, but its existence remains disputed; experiments conducted in 2008 could not replicate the compound.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
The fluoronickelates are a class of chemical compounds containing an anion with nickel at its core, surrounded by fluoride ions which act as ligands. This makes it a fluoroanion. The nickel atom can be in a range of oxidation states from +2, +3 to +4. The hexafluoronickelate(IV)2− ion NiF62− contains nickel in the maximal +4 state, and is in octahedral coordination by the fluoride atoms. It forms a commercially available salt Potassium hexafluoronickelate(IV) K2NiF6. Solid double salts can also contain tetrafluoronickelate NiF4 eg K2NiF4.
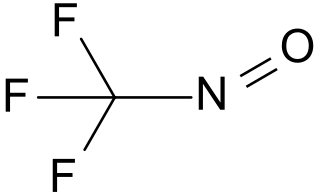
Trifluoronitrosomethane is a toxic organic compound consisting of a trifluoromethyl group covalently bound to a nitroso group. Its distinctive deep blue color is unusual for a gas.
Thullium(III) fluoride is an inorganic compound with the chemical formula TmF3.

Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3.
Alan K. Brisdon is a British chemist and a Senior Lecturer in the Department of Chemistry at The University of Manchester. His research in general is based on fluorine chemistry, including on HCFCs, fluorine-containing organometallic systems, fluorophosphines and fluorine-containing materials, such as ionic liquids and fluorographenes.















