
The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis; a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene INSR, from which alternate splicing during transcription results in either IR-A or IR-B isoforms. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon combination are ultimately capable of homo or hetero-dimerisation to produce the ≈320 kDa disulfide-linked transmembrane insulin receptor.
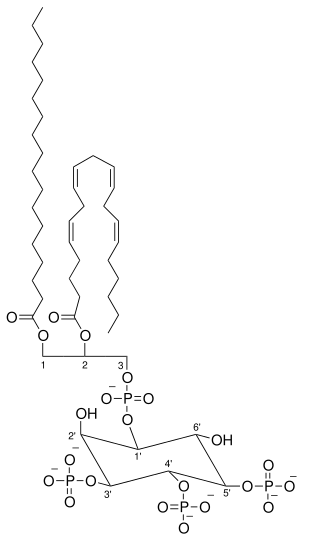
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.
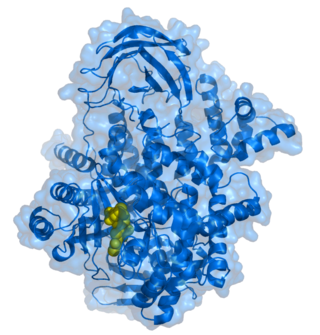
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

The erythropoietin receptor (EpoR) is a protein that in humans is encoded by the EPOR gene. EpoR is a 52 kDa peptide with a single carbohydrate chain resulting in an approximately 56–57 kDa protein found on the surface of EPO responding cells. It is a member of the cytokine receptor family. EpoR pre-exists as dimers. These dimers were originally thought to be formed by extracellular domain interactions, however, it is now assumed that it is formed by interactions of the transmembrane domain and that the original structure of the extracellular interaction site was due to crystallisation conditions and does not depict the native conformation. Binding of a 30 kDa ligand erythropoietin (Epo), changes the receptor's conformational change, resulting in the autophosphorylation of Jak2 kinases that are pre-associated with the receptor. At present, the best-established function of EpoR is to promote proliferation and rescue of erythroid progenitors from apoptosis.

Growth factor receptor-bound protein 2, also known as Grb2, is an adaptor protein involved in signal transduction/cell communication. In humans, the GRB2 protein is encoded by the GRB2 gene.

Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the PTPN11 gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2.
CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.

Lymphocyte cytosolic protein 2, also known as LCP2 or SLP-76, is a signal-transducing adaptor protein expressed in T cells and myeloid cells and is important in the signaling of T-cell receptors (TCRs). As an adaptor protein, SLP-76 does not have catalytic functions, primarily binding other signaling proteins to form larger signaling complexes. It is a key component of the signaling pathways of receptors with immunoreceptor tyrosine-based activation motifs (ITAMs) such as T-cell receptors, its precursors, and receptors for the Fc regions of certain antibodies. SLP-76 is expressed in T-cells and related lymphocytes like natural killer cells.

GRB2-associated-binding protein 2 also known as GAB2 is a protein that in humans is encoded by the GAB2 gene.
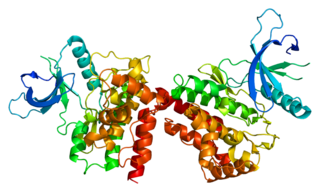
Janus kinase 2 is a non-receptor tyrosine kinase. It is a member of the Janus kinase family and has been implicated in signaling by members of the type II cytokine receptor family, the GM-CSF receptor family, the gp130 receptor family, and the single chain receptors.

Phosphatidylinositol 3-kinase regulatory subunit alpha is an enzyme that in humans is encoded by the PIK3R1 gene.
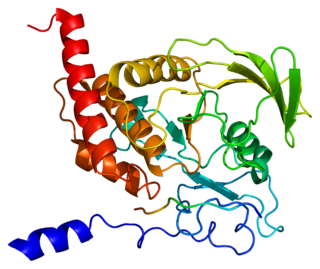
Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP-1), is an enzyme that in humans is encoded by the PTPN6 gene.

Cbl is a mammalian gene family. CBL gene, a part of the Cbl family, encodes the protein CBL which is an E3 ubiquitin-protein ligase involved in cell signalling and protein ubiquitination. Mutations to this gene have been implicated in a number of human cancers, particularly acute myeloid leukaemia.

Crk-like protein is a protein that in humans is encoded by the CRKL gene.

Docking protein 1 is a protein that in humans is encoded by the DOK1 gene.

SH2-domain containing Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 2 is an enzyme that in humans is encoded by the INPPL1 gene.

SH2 domain-containing adapter protein B is a protein that in humans is encoded by the SHB gene.

Docking protein 2 is a protein that in humans is encoded by the DOK2 gene.
Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase is an enzyme with systematic name 1-phosphatidyl-1D-myo-inositol-3,4,5-trisphosphate 5-phosphohydrolase, that has two isoforms: SHIP1 and SHIP2 (INPPL1).






















