
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses.

Strontianite (SrCO3) is an important raw material for the extraction of strontium. It is a rare carbonate mineral and one of only a few strontium minerals. It is a member of the aragonite group.

Anglesite is a lead sulfate mineral with the chemical formula PbSO4. It occurs as an oxidation product of primary lead sulfide ore, galena. Anglesite occurs as prismatic orthorhombic crystals and earthy masses, and is isomorphous with barite and celestine. It contains 74% of lead by mass and therefore has a high specific gravity of 6.3. Anglesite's color is white or gray with pale yellow streaks. It may be dark gray if impure.

Alstonite, also known as bromlite, is a low temperature hydrothermal mineral that is a rare double carbonate of calcium and barium with the formula BaCa(CO
3)
2, sometimes with some strontium. Barytocalcite and paralstonite have the same formula but different structures, so these three minerals are said to be trimorphous. Alstonite is triclinic but barytocalcite is monoclinic and paralstonite is trigonal. The species was named Bromlite by Thomas Thomson in 1837 after the Bromley-Hill mine, and alstonite by August Breithaupt of the Freiberg Mining Academy in 1841, after Alston, Cumbria, the base of operations of the mineral dealer from whom the first samples were obtained by Thomson in 1834. Both of these names have been in common use.

Barytocalcite is an anhydrous barium calcium carbonate mineral with the chemical formula BaCa(CO3)2. It is trimorphous with alstonite and paralstonite, that is to say the three minerals have the same formula but different structures. Baryte and quartz pseudomorphs after barytocalcite have been observed.

Linarite is a somewhat rare, crystalline mineral that is known among mineral collectors for its unusually intense, pure blue color. It is formed by the oxidation of galena and chalcopyrite and other copper sulfides. It is a combined copper lead sulfate hydroxide with formula PbCuSO4(OH)2. Linarite occurs as monoclinic prismatic to tabular crystals and irregular masses. It is easily confused with azurite, but does not react with dilute hydrochloric acid as azurite does. It has a Mohs hardness of 2.5 and a specific gravity of 5.3 – 5.5.

Vauxite is a phosphate mineral with the chemical formula Fe2+Al2(PO4)2(OH)2·6(H2O). It belongs to the laueite – paravauxite group, paravauxite subgroup, although Mindat puts it as a member of the vantasselite Al4(PO4)3(OH)3·9H2O group. There is no similarity in structure between vauxite and paravauxite Fe2+Al2(PO4)2(OH)2·8H2O or metavauxite Fe3+Al2(PO4)2(OH)2·8H2O, even though they are closely similar chemically and all minerals occur together as secondary minerals. Vauxite was named in 1922 for George Vaux Junior (1863–1927), an American attorney and mineral collector.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.

Mendipite is a rare mineral that was named in 1939 for the locality where it is found, the Mendip Hills in Somerset, England. It is an oxyhalide of lead with formula Pb3Cl2O2.

Sarabauite (sar-a-bau'-ite) is a red monoclinic sulfide mineral with the chemical formula: CaSb10O10S6.

Tsumcorite is a rare hydrated lead arsenate mineral that was discovered in 1971, and reported by Geier, Kautz and Muller. It was named after the TSUMeb CORporation mine at Tsumeb, in Namibia, in recognition of the Corporation's support for mineralogical investigations of the orebody at its Mineral Research Laboratory.

Brianyoungite is a secondary zinc carbonate mineral. The Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) classifies it as a carbonate with the formula Zn3(CO3)(OH)4, but sulfate groups SO4 also occupy the carbonate CO3 positions, in the ratio of about one sulfate to three carbonates, so other sources give the formula as Zn3(CO3,SO4)(OH)4, and Gaines et al. classify the mineral as a compound carbonate. It is similar in appearance to hydrozincite, another zinc carbonate. It was discovered in 1991 and designated IMA1991-053. In 1993 it was named "brianyoungite" after Brian Young (born 1947), a field geologist with the British Geological Survey, who provided the first specimens.

Köttigite is a rare hydrated zinc arsenate which was discovered in 1849 and named by James Dwight Dana in 1850 in honour of Otto Friedrich Köttig (1824–1892), a German chemist from Schneeberg, Saxony, who made the first chemical analysis of the mineral. It has the formula Zn3(AsO4)2·8H2O and it is a dimorph of metaköttigite, which means that the two minerals have the same formula, but a different structure: köttigite is monoclinic and metaköttigite is triclinic. There are several minerals with similar formulae but with other cations in place of the zinc. Iron forms parasymplesite Fe2+3(AsO4)2·8H2O; cobalt forms the distinctively coloured pinkish purple mineral erythrite Co3(AsO4)2·8H2O and nickel forms annabergite Ni3(AsO4)2·8H2O. Köttigite forms series with all three of these minerals and they are all members of the vivianite group.

Carminite (PbFe3+2(AsO4)2(OH)2) is an anhydrous arsenate mineral containing hydroxyl. It is a rare secondary mineral that is structurally related to palermoite (Li2SrAl4(PO4)4(OH)4). Sewardite (CaFe3+2(AsO4)2(OH)2) is an analogue of carminite, with calcium in sewardite in place of the lead in carminite. Mawbyite is a dimorph (same formula, different structure) of carminite; mawbyite is monoclinic and carminite is orthorhombic. It has a molar mass of 639.87 g. It was discovered in 1850 and named for the characteristic carmine colour.

Serpierite (Ca(Cu,Zn)4(SO4)2(OH)6·3H2O) is a rare, sky-blue coloured hydrated sulfate mineral, often found as a post-mining product. It is a member of the devilline group, which has members aldridgeite (Cd,Ca)(Cu,Zn)4(SO4)2(OH)6·3H2O, campigliaite Cu4Mn2+(SO4)2(OH)6·4H2O, devilline CaCu4(SO4)2(OH)6·3H2O, kobyashevite Cu5(SO4)2(OH)6·4H2O, lautenthalite PbCu4(SO4)2(OH)6·3H2O and an unnamed dimorph of devilline. It is the calcium analogue of aldridgeite and it is dimorphous with orthoserpierite CaCu4(SO4)2(OH)6·3H2O.

Mottramite is an orthorhombic anhydrous vanadate hydroxide mineral, PbCu(VO4)(OH), at the copper end of the descloizite subgroup. It was formerly called cuprodescloizite or psittacinite (this mineral characterized in 1868 by Frederick Augustus Genth). Duhamelite is a calcium- and bismuth-bearing variety of mottramite, typically with acicular habit.

Talmessite is a hydrated calcium magnesium arsenate, often with significant amounts of cobalt or nickel. It was named in 1960 for the type locality, the Talmessi mine, Anarak district, Iran. It forms a series with β-Roselite, where cobalt replaces some of the magnesium, and with gaitite, where zinc replaces the magnesium. All these minerals are members of the fairfieldite group. Talmessite is dimorphic with wendwilsonite.

Macphersonite, Pb4(SO4)(CO3)2 (OH)2, is a carbonate mineral that is trimorphous with leadhillite and susannite. Macphersonite is generally white, colorless, or a pale amber in color and has a white streak. It crystallizes in the orthorhombic system with a space group of Pcab. It is fairly soft mineral that has a high specific gravity.
Scotlandite is a sulfite mineral first discovered in a mine at Leadhills in South Lanarkshire, Scotland, an area known to mineralogists and geologists for its wide range of different mineral species found in the veins that lie deep in the mine shafts. This specific mineral is found in the Susanna vein of Leadhills, where the crystals are formed as chisel-shaped or bladed. Scotlandite was actually the first naturally occurring sulfite, which has the ideal chemical formula of PbSO3. The mineral has been approved by the Commission on New Minerals and Mineral Names, IMA, to be named scotlandite for Scotland.
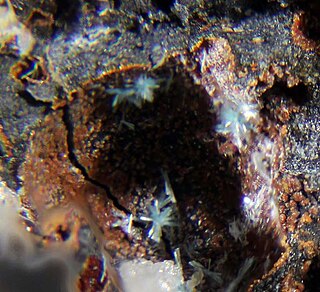
Mammothite is a mineral found in the Mammoth mine in Tiger, Arizona and also in Laurium, Attika, Greece. This mineral was named in 1985 by Donald R. Peacor, Pete J. Dunn, G. Schnorrer-Köhler, and Richard A. Bideaux, for the Mammoth vein (one of the two main veins in the mine) and the town of Mammoth, Arizona, which was named for the mine. The mammothite that is found in Arizona exist as euhedral crystals imbedded in micro granular, white colored anglesite with a saccharoidal texture. The associated minerals include phosgenite, wulfenite, leadhillite and caledonite. In Greece, the mammothite exists as small euhedral crystals and also as microscopic rock cavities lined with projecting crystals within the slags. The associated minerals here are cerussite, phosgenite and matlockite. The ideal chemical formula for mammothite is Pb6Cu4AlSb5+O2(OH)16Cl4(SO4)2.





















