
Lipoxygenases (LOX) are a family of (non-heme) iron-containing enzymes, more specifically oxidative enzymes, most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.

2,4 Dienoyl-CoA reductase also known as DECR1 is an enzyme which in humans is encoded by the DECR1 gene which resides on chromosome 8. This enzyme catalyzes the following reactions

Mead acid is an omega-9 fatty acid, first characterized by James F. Mead. As with some other omega-9 polyunsaturated fatty acids, animals can make Mead acid de novo. Its elevated presence in the blood is an indication of essential fatty acid deficiency. Mead acid is found in large quantities in cartilage.
In enzymology, a linoleate 11-lipoxygenase (EC 1.13.11.45) is an enzyme that catalyzes the chemical reaction
In enzymology, a linoleate diol synthase (EC 1.13.11.44) is an enzyme that catalyzes the chemical reaction
In enzymology, an ent-copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction:
The enzyme hydroperoxide dehydratase (EC 4.2.1.92) catalyzes the chemical reaction

Epidermis-type lipoxygenase 3 is a member of the lipoxygenase family of enzymes; in humans, it is encoded by the ALOXE3 gene. This gene is located on chromosome 17 at position 13.1 where it forms a cluster with two other lipoxygenases, ALOX12B and ALOX15B. Among the human lipoxygenases, ALOXE3 is most closely related in amino acid sequence to ALOX12B. ALOXE3, ALOX12B, and ALOX15B are often classified as epidermal lipoxygenases, in distinction to the other three human lipoxygenases, because they were initially defined as being highly or even exclusively expressed and functioning in skin. The epidermis-type lipoxygenases are now regarded as a distinct subclass within the multigene family of mammalian lipoxygenases with mouse Aloxe3 being the ortholog to human ALOXE3, mouse Alox12b being the ortholog to human ALOX12B, and mouse Alox8 being the ortholog to human ALOX15B [supplied by OMIM]. ALOX12B and ALOXE3 in humans, Alox12b and Aloxe3 in mice, and comparable orthologs in other in other species are proposed to act sequentially in a multistep metabolic pathway that forms products that are structurally critical for creating and maintaining the skin's water barrier function.
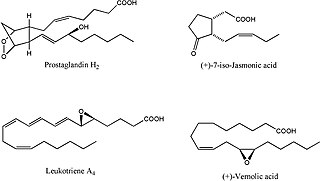
Oxylipins constitute a family of oxygenated natural products which are formed from fatty acids by pathways involving at least one step of dioxygen-dependent oxidation. These small polar lipid compounds are metabolites of polyunsaturated fatty acids (PUFAs) including omega-3 fatty acids and omega-6 fatty acids. Oxylipins are formed by enyzmatic or non-enzymatic oxidation of PUFAs.
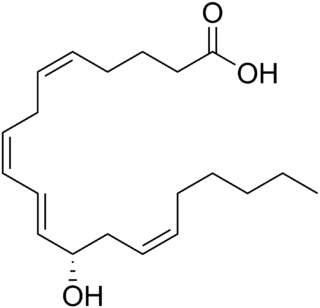
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.

Soluble epoxide hydrolase (sEH) is a bifunctional enzyme that in humans is encoded by the EPHX2 gene. sEH is a member of the epoxide hydrolase family. This enzyme, found in both the cytosol and peroxisomes, binds to specific epoxides and converts them to the corresponding diols. A different region of this protein also has lipid-phosphate phosphatase activity. Mutations in the EPHX2 gene have been associated with familial hypercholesterolemia.
Plant seed peroxygenase (EC 1.11.2.3, plant peroxygenase, soybean peroxygenase) is an enzyme with systematic name substrate:hydroperoxide oxidoreductase (RH-hydroxylating or epoxidising). This enzyme catalyses the following chemical reaction
5,8-linoleate diol synthase may refer to:
Linolenate 9R-lipoxygenase (EC 1.13.11.61, NspLOX, (9R)-LOX, linoleate 9R-dioxygenase) is an enzyme with systematic name alpha-linolenate:oxygen (9R)-oxidoreductase. This enzyme catalyses the following chemical reaction
Linoleate 10R-lipoxygenase (EC 1.13.11.62, 10R-DOX, (10R)-dioxygenase, 10R-dioxygenase) is an enzyme with systematic name linoleate:oxygen (10R)-oxidoreductase. This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8R-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -5,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
9,12-octadecadienoate 8-hydroperoxide 8S-isomerase is an enzyme with systematic name (8R,9Z,12Z)-8-hydroperoxyoctadeca-9,12-dienoate hydroxymutase ( -7,8-dihydroxyoctadeca-9,12-dienoate-forming). This enzyme catalyses the following chemical reaction
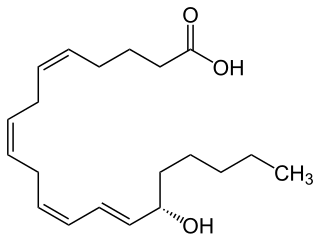
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
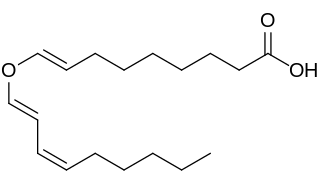
Divinylether fatty acids contain a fatty acid chemically combined with a doubly unsaturated carbon chain linked by an oxygen atom (ether). Fatty acid hydroperoxides generated by plant lipoxygenases from linoleic and linolenic acids are known to serve as substrates for a divinyl ether synthase which produces divinyl ether fatty acids. Up to date divinyl ethers were detected only within the plant kingdom.

In biochemistry, non-heme iron proteins describe families of enzymes that utilize iron at the active site but lack heme cofactors. Iron-sulfur proteins, including those that are enzymes, are not included in this definition.










