Related Research Articles

The Controlled Substances Act (CSA) is the statute establishing federal U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs.

The term narcotic originally referred medically to any psychoactive compound with numbing or paralyzing properties. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine.

Methylphenidate, sold under the brand names Ritalin and Concerta among others, is a central nervous system (CNS) stimulant used medically to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, narcolepsy. It is a primary medication for ADHD ; it may be taken by mouth or applied to the skin, and different formulations have varying durations of effect, commonly ranging from 2–4 hours. Methylphenidate's efficacy as a athletic performance enhancer, cognitive enhancer, aphrodisiac, and euphoriant is somewhat supported by research. However, the manner in which methylphenidate is used for these purposes can result in severe unintended side effects.

Propylhexedrine, sold under the brand name Benzedrex, is a nasal decongestant, appetite suppressant, and psychostimulant medication. It is used medicinally for relief of congestion due to colds, allergies and allergic rhinitis.

HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 has a binding affinity of 0.061nM at CB1 and 0.52nM at CB2 in cloned human cannabinoid receptors compared to Delta-9-THC of 40.7nM at CB1. HU-210 is the (–)-1,1-dimethylheptyl analog of 11-hydroxy- Δ8- tetrahydrocannabinol; in some references it is called 1,1-dimethylheptyl- 11-hydroxytetrahydrocannabinol. The abbreviation "HU" stands for Hebrew University.

Amobarbital is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate. If amobarbital is taken for extended periods of time, physiological and psychological dependence can develop. Amobarbital withdrawal mimics delirium tremens and may be life-threatening. Amobarbital was manufactured by Eli Lilly and Company in the US under the brand name Amytal in bright blue bullet shaped capsules or pink tablets containing 50, 100, or 200 milligrams of the drug. The drug was also manufactured generically. Amobarbital was widely misused, known as "Blue Heavens" on the street. Amytal, as well as Tuinal, a combination drug containing equal quantities of secobarbital and amobarbital, were both manufactured by Eli Lilly until the late-1990s. However, as the popularity of benzodiazepines increased, prescriptions for these medications became increasingly rare beginning in the mid to late-1980s.
Pentobarbital is a short-acting barbiturate typically used as a sedative, a preanesthetic, and to control convulsions in emergencies. It can also be used for short-term treatment of insomnia but has been largely replaced by the benzodiazepine family of drugs.

Tiletamine is a dissociative anesthetic and pharmacologically classified as an NMDA receptor antagonist. It is related chemically to ketamine. Tiletamine hydrochloride exists as odorless white crystals.

Zolazepam (Flupyrazapon) is a pyrazolodiazepinone derivative structurally related to the benzodiazepine drugs, which is used as an anaesthetic for a wide range of animals in veterinary medicine. Zolazepam is usually administered in combination with other drugs such as the NMDA antagonist tiletamine or the α2 adrenergic receptor agonist xylazine, depending on what purpose it is being used for. It is around four times the potency of diazepam but it is both water-soluble and un-ionized at physiological pH meaning that its onset is very fast.
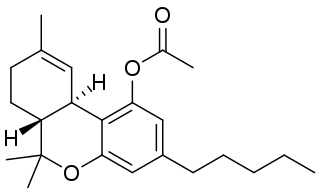
THC acetate ester is the acetate ester of THC. It is a potent synthetic homologue and a cannabinoid known to exhibit hallucinogenic effects that have been described as 300% more potent than THC. It is structurally identical to THC except for the addition of an ester group. It has been described by groups of varying expertise as having spiritual and psychedelic effects.
The Opium Law is the section of the Dutch law which covers nearly all psychotropic drugs.

The Convention on Psychotropic Substances of 1971 is a United Nations treaty designed to control psychoactive drugs such as amphetamine-type stimulants, barbiturates, benzodiazepines, and psychedelics signed in Vienna, Austria on 21 February 1971. The Single Convention on Narcotic Drugs of 1961 did not ban the many newly discovered psychotropics, since its scope was limited to drugs with cannabis, coca and opium-like effects.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.

Isomethadone (INN, BAN; trade nameLiden; also known as isoamidone) is a synthetic opioid analgesic and antitussive related to methadone that was used formerly as a pharmaceutical drug but is now no longer marketed. Isomethadone was used as both an analgesic and antitussive. It binds to and activates both the μ- and δ-opioid receptors, with the (S)-isomer being the more potent of its two enantiomers. Isomethadone is a Schedule II controlled substance in the United States, with an ACSCN of 9226 and a 2014 aggregate manufacturing quota of 5 g. The salts in use are the hydrobromide (HBr, free base conversion ratio 0.793), hydrochloride (HCl, 0.894), and HCl monohydrate (0.850). Isomethadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.
The major drug laws of India are the Narcotic Drugs and Psychotropic Substances Act (1985) and the Prevention of Illicit Trafficking in Narcotic Drugs and Psychotropic Substances Act (1988).
Schedule X is a class of prescription drugs in India appearing as an appendix to the Drugs and Cosmetics Rules introduced in 1945. These are drugs which cannot be purchased over the counter without the prescription of a qualified doctor. Also, the retailer has to preserve the prescription for a period of two years.

Thenylfentanyl is an analogue of fentanyl where the phenethylamine side-chain has been replaced by a thiophenylmethyl group. It was temporarily scheduled by the Drug Enforcement Administration in 1985, due to fears it would be used as a designer drug. But in 2010 the DEA acknowledged it was essentially inactive. Subsequently, the substance was since deregulated.
References
- 1 2 21 CFR 1308.12 Archived 2015-08-04 at the Wayback Machine (CSA Sched II) with changes through 77 FR 64032 (Oct 18, 2012). Retrieved September 6, 2013.
- ↑ retrieved October 7, 2007
- ↑ "Schedules of Controlled Substances; Transfer of Levo-acetylmethadol from Schedule I into Schedule II" (PDF). Isomer Design. Drug Enforcement Administration. August 18, 1993. Archived from the original (PDF) on July 28, 2019. Retrieved January 16, 2023.
- ↑ "Schedules of Controlled Substances: Placement of Remifentanil Into Schedule II". Federal Register . Drug Enforcement Administration. November 5, 1996. Retrieved January 16, 2023.
- ↑ "Schedules of Controlled Substances; Rescheduling of Sufentanil into Schedule II" (PDF). Isomer Design. Drug Enforcement Administration. May 25, 1984. Archived (PDF) from the original on July 29, 2019. Retrieved January 16, 2023.
- ↑ "Schedules of Controlled Substances: Placement of Tapentadol Into Schedule II". Federal Register . Drug Enforcement Administration. May 21, 2009. Retrieved January 16, 2023.
- ↑ "Amphetamine, Methamphetamine, and Optical Isomers" (PDF). Isomer Design. July 7, 1971. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Amphetamine, Methamphetamine, and Optical Isomers" (PDF). Isomer Design. July 7, 1971. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Phenmetrazine and its salts, and Methylphenidate" (PDF). Isomer Design. Bureau of Narcotics and Dangerous Drugs. October 28, 1971. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Phenmetrazine and its salts, and Methylphenidate" (PDF). Isomer Design. Bureau of Narcotics and Dangerous Drugs. October 28, 1971. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Schedules of Controlled Substances: Placement of Lisdexamfetamine Into Schedule II". Federal Register. Drug Enforcement Administration. May 3, 2007. Archived from the original on January 16, 2023. Retrieved January 16, 2023.
- ↑ "Schedule II Control of Amobarbital, Pentobarbital, Secobarbital, and their salts" (PDF). Drug Enforcement Administration. November 13, 1973. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Schedules of Controlled Substances: Transfer of Glutethimide from Schedule III to Schedule II" (PDF). Isomer Design. Drug Enforcement Administration. March 21, 1991. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Schedule II Control of Amobarbital, Pentobarbital, Secobarbital, and their salts" (PDF). Drug Enforcement Administration. November 13, 1973. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Placement of Phencyclidine in Schedule II" (PDF). Isomer Design. Drug Enforcement Administration. January 25, 1978. Archived (PDF) from the original on March 3, 2023. Retrieved January 16, 2023.
- ↑ "Schedule II Control of Amobarbital, Pentobarbital, Secobarbital, and their salts" (PDF). Drug Enforcement Administration. November 13, 1973. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Schedules of Controlled Substances; Schedule II Placement of Phenylacetone; (Phenyl-2-propanone, P2P, methyl benzyl ketone, benzyl methyl ketone)" (PDF). Isomer Design. Drug Enforcement Administration. February 11, 1980. Archived (PDF) from the original on March 3, 2022. Retrieved January 16, 2023.
- ↑ "Control of Immediate Precursor Used in the Illicit Manufacture of Fentanyl as a Schedule II Controlled Substance". Federal Register. Drug Enforcement Administration. June 29, 2010. Archived from the original on January 16, 2023. Retrieved January 16, 2023.