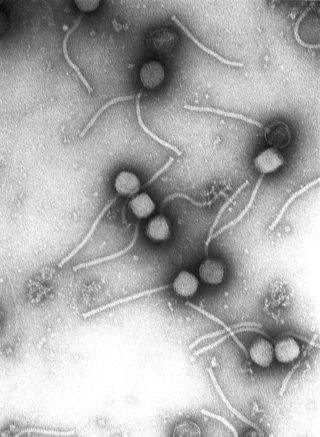
Virology is the scientific study of biological viruses. It is a subfield of microbiology that focuses on their detection, structure, classification and evolution, their methods of infection and exploitation of host cells for reproduction, their interaction with host organism physiology and immunity, the diseases they cause, the techniques to isolate and culture them, and their use in research and therapy.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.

Picornaviruses are a group of related nonenveloped RNA viruses which infect vertebrates including fish, mammals, and birds. They are viruses that represent a large family of small, positive-sense, single-stranded RNA viruses with a 30 nm icosahedral capsid. The viruses in this family can cause a range of diseases including the common cold, poliomyelitis, meningitis, hepatitis, and paralysis.
Polyomaviridae is a family of viruses whose natural hosts are primarily mammals and birds. As of 2024, there are eight recognized genera. 14 species are known to infect humans, while others, such as Simian Virus 40, have been identified in humans to a lesser extent. Most of these viruses are very common and typically asymptomatic in most human populations studied. BK virus is associated with nephropathy in renal transplant and non-renal solid organ transplant patients, JC virus with progressive multifocal leukoencephalopathy, and Merkel cell virus with Merkel cell cancer.
Lyssavirus is a genus of RNA viruses in the family Rhabdoviridae, order Mononegavirales. Mammals, including humans, can serve as natural hosts. The genus Lyssavirus includes the rabies virus traditionally associated with the disease of the same name.

Geminiviridae is a family of plant viruses that encode their genetic information on a circular genome of single-stranded (ss) DNA. There are 520 species in this family, assigned to 14 genera. Diseases associated with this family include: bright yellow mosaic, yellow mosaic, yellow mottle, leaf curling, stunting, streaks, reduced yields. They have single-stranded circular DNA genomes encoding genes that diverge in both directions from a virion strand origin of replication. According to the Baltimore classification they are considered class II viruses. It is the largest known family of single stranded DNA viruses.

Sheeppox is a highly contagious disease of sheep caused by a poxvirus different from the benign orf. This virus is in the family Poxviridae and genus Capripoxvirus. Sheeppox virus (SPV) is the most severe of all the animal pox diseases and can result in some of the most significant economic consequences due to poor wool and leather quality.

Iridoviridae is a family of viruses with double-stranded DNA genomes. Amphibians, fish, and invertebrates such as arthropods serve as natural hosts. There are currently 22 species in this family, divided among two subfamilies and seven genera.

Ascoviridae is a family of double strand DNA viruses that infect primarily invertebrates, mainly noctuids and spodoptera species; it contains two genera, Ascovirus, which contains three species, and Toursvirus with a single species Diadromus pulchellus toursvirus.

Ranavirus is a genus of viruses in the family Iridoviridae. There are six other genera of viruses within the family Iridoviridae, but Ranavirus is the only one that includes viruses that are infectious to amphibians and reptiles. Additionally, it is one of the three genera within this family which infect teleost fishes, along with Lymphocystivirus and Megalocytivirus.
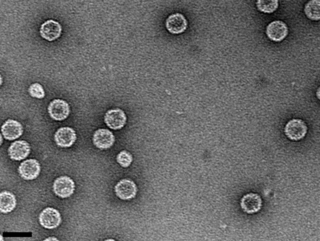
Picobirnavirus is a genus of double-stranded RNA viruses. It is the only genus in the family Picobirnaviridae. Although amniotes, especially mammals, were thought to serve as hosts, it has been recently suggested that these viruses might infect bacteria and possibly some other invertebrates. If they do infect bacteria, then they are Bacteriophages. There are three species in this genus. Associated symptoms include gastroenteritis in animals and humans, though the disease association is unclear.
Megalocytivirus is a genus of viruses in the family Iridoviridae and one of three genera within this family which infect teleost fishes, along with Lymphocystivirus and Ranavirus. Megalocytiviruses are an emerging group of closely related dsDNA viruses which cause systemic infections in a wide variety of wild and cultured fresh and saltwater fishes. Megalocytivirus outbreaks are of considerable economic importance in aquaculture, as epizootics can result in moderate fish loss or mass mortality events of cultured fishes.
Hytrosaviridae is a family of double-stranded DNA viruses that infect insects. The name is derived from Hytrosa, sigla from the Greek Hypertrophia for 'hypertrophy' and 'sialoadenitis' for 'salivary gland inflammation.'
Tilapia tilapinevirus, or Tilapia lake virus (TiLV), is a negative-strand RNA virus that infects both wild and aquacultured populations of tilapia. It is the only species in the monotypic genus Tilapinevirus, which in turn is the only genus in the family Amnoonviridae. Thus far it has been recorded in various regions across Asia, Africa, and South America. The virus was first discovered and identified in 2014 when the Sea of Galilee in Israel experienced a major noticeable decline in tilapia catch quantities.

Avian metaavulavirus 2, formerly Avian paramyxovirus 2, is a species of virus belonging to the family Paramyxoviridae and genus Metaavulavirus. The virus is a negative strand RNA virus containing a monopartite genome. Avian metaavulavirus 2 is one of nine species belonging to the genus Metaavulavirus. The most common serotype of Avulavirinae is serotype 1, the cause of Newcastle disease (ND). Avian metaavulavirus 2 has been known to cause disease, specifically mild respiratory infections in domestic poultry, including turkeys and chickens, and has many economic effects on egg production and poultry industries. The virus was first isolated from a strain in Yucaipa, California in 1956. Since then, other isolates of the virus have been isolated worldwide.
Cyvirus anguillidallo1, also known as Anguillid herpesvirus 1 (AngHV-1) is a species of virus in the genus Cyprinivirus, family Alloherpesviridae, and order Herpesvirales.
Acipenserid herpesvirus 2 (AciHV-2) is a virus in the genus Ictalurivirus, family Alloherpesviridae, and order Herpesvirales. It infects sturgeon of the family Acipenseridae.
Salmonid herpesvirus 3 (SalHV-3) is a species of virus in the genus Salmonivirus, family Alloherpesviridae, and order Herpesvirales.
Nucleocytoviricota is a phylum of viruses. Members of the phylum are also known as the nucleocytoplasmic large DNA viruses (NCLDV), which serves as the basis of the name of the phylum with the suffix -viricota for virus phylum. These viruses are referred to as nucleocytoplasmic because they are often able to replicate in both the host's cell nucleus and cytoplasm.

Thaspiviridae is a family of incertae sedis spindle-shaped viruses. The family contains a single genus, Nitmarvirus, which contains a single species, Nitmarvirus NSV1.












