
The green sulfur bacteria are a phylum, Chlorobiota, of obligately anaerobic photoautotrophic bacteria that metabolize sulfur.

Anammox, an abbreviation for "anaerobic ammonium oxidation", is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water.
The Desulfobacteraceae are a family of Thermodesulfobacteriota. They reduce sulfates to sulfides to obtain energy and are strictly anaerobic. They have a respiratory and fermentative type of metabolism. Some species are chemolithotrophic and use inorganic materials to obtain energy and use hydrogen as their electron donor.
Sulfur-reducing bacteria are microorganisms able to reduce elemental sulfur (S0) to hydrogen sulfide (H2S). These microbes use inorganic sulfur compounds as electron acceptors to sustain several activities such as respiration, conserving energy and growth, in absence of oxygen. The final product of these processes, sulfide, has a considerable influence on the chemistry of the environment and, in addition, is used as electron donor for a large variety of microbial metabolisms. Several types of bacteria and many non-methanogenic archaea can reduce sulfur. Microbial sulfur reduction was already shown in early studies, which highlighted the first proof of S0 reduction in a vibrioid bacterium from mud, with sulfur as electron acceptor and H
2 as electron donor. The first pure cultured species of sulfur-reducing bacteria, Desulfuromonas acetoxidans, was discovered in 1976 and described by Pfennig Norbert and Biebel Hanno as an anaerobic sulfur-reducing and acetate-oxidizing bacterium, not able to reduce sulfate. Only few taxa are true sulfur-reducing bacteria, using sulfur reduction as the only or main catabolic reaction. Normally, they couple this reaction with the oxidation of acetate, succinate or other organic compounds. In general, sulfate-reducing bacteria are able to use both sulfate and elemental sulfur as electron acceptors. Thanks to its abundancy and thermodynamic stability, sulfate is the most studied electron acceptor for anaerobic respiration that involves sulfur compounds. Elemental sulfur, however, is very abundant and important, especially in deep-sea hydrothermal vents, hot springs and other extreme environments, making its isolation more difficult. Some bacteria – such as Proteus, Campylobacter, Pseudomonas and Salmonella – have the ability to reduce sulfur, but can also use oxygen and other terminal electron acceptors.
The oxidase test is used to determine if an organism possesses the cytochrome c oxidase enzyme. The test is used as an aid for the differentiation of Neisseria, Moraxella, Campylobacter and Pasteurella species. It is also used to differentiate pseudomonads from related species.

Beggiatoa is a genus of Gammaproteobacteria belonging to the order Thiotrichales, in the Pseudomonadota phylum. This genus was one of the first bacteria discovered by Ukrainian botanist Sergei Winogradsky. During his research in Anton de Bary's laboratory of botany in 1887, he found that Beggiatoa oxidized hydrogen sulfide (H2S) as an energy source, forming intracellular sulfur droplets, with oxygen as the terminal electron acceptor and CO2 used as a carbon source. Winogradsky named it in honor of the Italian doctor and botanist Francesco Secondo Beggiato (1806 - 1883), from Venice. Winogradsky referred to this form of metabolism as "inorgoxidation" (oxidation of inorganic compounds), today called chemolithotrophy. These organisms live in sulfur-rich environments such as soil, both marine and freshwater, in the deep sea hydrothermal vents and in polluted marine environments. The finding represented the first discovery of lithotrophy. Two species of Beggiatoa have been formally described: the type species Beggiatoa alba and Beggiatoa leptomitoformis, the latter of which was only published in 2017. This colorless and filamentous bacterium, sometimes in association with other sulfur bacteria (for example the genus Thiothrix), can be arranged in biofilm visible to the naked eye formed by a very long white filamentous mat, the white color is due to the stored sulfur. Species of Beggiatoa have cells up to 200 µm in diameter and they are one of the largest prokaryotes on Earth.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
Paracoccus denitrificans, is a coccoid bacterium known for its nitrate reducing properties, its ability to replicate under conditions of hypergravity and for being a relative of the eukaryotic mitochondrion.
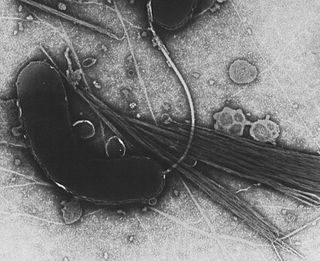
Gammaproteobacteria is a class of bacteria in the phylum Pseudomonadota. It contains about 250 genera, which makes it the most genus-rich taxon of the Prokaryotes. Several medically, ecologically, and scientifically important groups of bacteria belong to this class. It is composed by all Gram-negative microbes and is the most phylogenetically and physiologically diverse class of Proteobacteria.
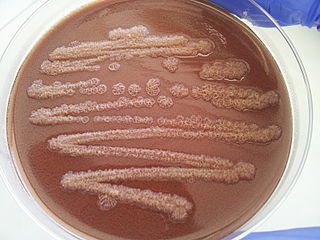
Pseudomonas stutzeri is a Gram-negative soil bacterium that is motile, has a single polar flagellum, and is classified as bacillus, or rod-shaped. While this bacterium was first isolated from human spinal fluid, it has since been found in many different environments due to its various characteristics and metabolic capabilities. P. stutzeri is an opportunistic pathogen in clinical settings, although infections are rare. Based on 16S rRNA analysis, this bacterium has been placed in the P. stutzeri group, to which it lends its name.
Chromohalobacter beijerinckii is a motile, rod-like, salt-loving, Gram-negative soil bacterium, 0.4–0.6 μm by 1.8–2.5 μm.

Thioploca is a genus of filamentous sulphur-oxidizing bacteria which occurs along 3,000 kilometres (1,900 mi) of coast off the west of South America. Was discovered in 1907 by R. Lauterborn classified as belonging to the order Thiotrichales, part of the Gammaproteobacteria. They inhabit as well marine as freshwater environments, with vast communities present off the Pacific coast of South America and other areas with a high organic matter sedimentation and bottom waters rich in nitrate and poor in oxygen. A large vacuole occupies more than 80% of their cellular volume and is used as a storage for nitrate. This nitrate is used for the sulphur oxidation, an important characteristic of the genus. Due to their unique size in diameters, ranging from 15-40 µm, they are considered part of the largest bacteria known. Because they use both sulfur and nitrogen compounds they may provide an important link between the nitrogen and sulphur cycles. They secrete a sheath of mucus which they use as a tunnel to travel between the sulfide containing sediment and the nitrate containing sea water.
"Candidatus Scalindua" is a bacterial genus, and a proposed member of the order Planctomycetales. These bacteria lack peptidoglycan in their cell wall and have a compartmentalized cytoplasm. They are ammonium oxidizing bacteria found in marine environments.
Geothrix fermentans is a rod-shaped, anaerobic bacterium. It is about 0.1 µm in diameter and ranges from 2-3 µm in length. Cell arrangement occurs singly and in chains. Geothrix fermentans can normally be found in aquatic sediments such as in aquifers. As an anaerobic chemoorganotroph, this organism is best known for its ability to use electron acceptors Fe(III), as well as other high potential metals. It also uses a wide range of substrates as electron donors. Research on metal reduction by G. fermentans has contributed to understanding more about the geochemical cycling of metals in the environment.
Acidithiobacillus caldus formerly belonged to the genus Thiobacillus prior to 2000, when it was reclassified along with a number of other bacterial species into one of three new genera that better categorize sulfur-oxidizing acidophiles. As a member of the Gammaproteobacteria class of Pseudomonadota, A. caldus may be identified as a Gram-negative bacterium that is frequently found in pairs. Considered to be one of the most common microbes involved in biomining, it is capable of oxidizing reduced inorganic sulfur compounds (RISCs) that form during the breakdown of sulfide minerals. The meaning of the prefix acidi- in the name Acidithiobacillus comes from the Latin word acidus, signifying that members of this genus love a sour, acidic environment. Thio is derived from the Greek word thios and describes the use of sulfur as an energy source, and bacillus describes the shape of these microorganisms, which are small rods. The species name, caldus, is derived from the Latin word for warm or hot, denoting this species' love of a warm environment.

Acidithiobacillus thiooxidans, formerly known as Thiobacillus thiooxidans until its reclassification into the newly designated genus Acidithiobacillus of the Acidithiobacillia subclass of Pseudomonadota, is a Gram-negative, rod-shaped bacterium that uses sulfur as its primary energy source. It is mesophilic, with a temperature optimum of 28 °C. This bacterium is commonly found in soil, sewer pipes, and cave biofilms called snottites. A. thiooxidans is used in the mining technique known as bioleaching, where metals are extracted from their ores through the action of microbes.
Macromonas is a genus in the phylum Pseudomonadota (Bacteria).
Xenophilus azovorans is a bacterium from the genus Xenophilus which has been isolated from soil in Switzerland.

Microbial oxidation of sulfur is the oxidation of sulfur by microorganisms to build their structural components. The oxidation of inorganic compounds is the strategy primarily used by chemolithotrophic microorganisms to obtain energy to survive, grow and reproduce. Some inorganic forms of reduced sulfur, mainly sulfide (H2S/HS−) and elemental sulfur (S0), can be oxidized by chemolithotrophic sulfur-oxidizing prokaryotes, usually coupled to the reduction of oxygen (O2) or nitrate (NO3−). Anaerobic sulfur oxidizers include photolithoautotrophs that obtain their energy from sunlight, hydrogen from sulfide, and carbon from carbon dioxide (CO2).
"Candidatus Brocadia" is a candidatus genus of bacteria, meaning that while it is well-characterized, it has not been grown as a pure culture yet. Due to this, much of what is known about Candidatus species has been discovered using culture-independent techniques such as metagenomic sequence analysis.








