Acrylonitrile is an organic compound with the formula CH2CHCN. It is a colorless volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile. It is an important monomer for the manufacture of useful plastics such as polyacrylonitrile. It is reactive and toxic at low doses. Acrylonitrile was first synthesized by the French chemist Charles Moureu (1863–1929) in 1893.

Chlorfenvinphos is the common name of an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was cancelled in 1991.

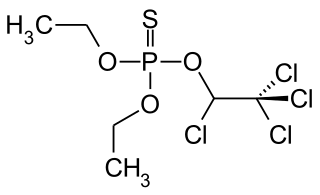
Ethion (C9H22O4P2S4) is an organophosphate insecticide. Ethion is known to affect a neural enzyme called acetylcholinesterase and prevent it from working.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.

Sudan I, is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.
4-Aminobiphenyl (4-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.

Benzotrichloride (BTC), also known as α,α,α-trichlorotoluene, phenyl chloroform or (trichloromethyl)benzene, is an organic compound with the formula C6H5CCl3. Benzotrichloride is an unstable, colorless (to yellowish), viscous, chlorinated hydrocarbon with a penetrating odor. Benzotrichloride is used extensively as a chemical intermediate for products of various classes, i.e. dyes and antimicrobial agents.

o-Toluidine (ortho-toluidine) is an organic compound with the chemical formula CH3C6H4NH2. It is the most important of the three isomeric toluidines. It is a colorless liquid although commercial samples are often yellowish. It is a precursor to the herbicides metolachlor and acetochlor.

1,1,2,2-tetrachloroethane (TeCA), also known as bonoform, cellon, or westron is a toxic, synthetic halogen rich alkane. It is colorless liquid and has a sweet odor. It is used as an industrial solvent or as a separation agent. TeCA can be inhaled, consumed or absorbed through the skin. After exposure, nausea, dizziness or even liver damage may occur.
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.

Hexachlorocyclopentadiene (HCCPD), also known as C-56, Graphlox, and HRS 1655, is an organochlorine compound with the formula C5Cl6. It is a precursor to pesticides, flame retardants, and dyes. It is a colourless liquid, although commercial samples appear lemon-yellow liquid sometimes with a bluish vapour. Many of its derivatives proved to be highly controversial, as studies showed them to be persistent organic pollutants. An estimated 270,000 tons were produced until 1976, and smaller amounts continue to be produced today. Two prominent manufacturers are Velsicol Chemical Corporation in the US and by Jiangsu Anpon Electrochemicals Co. in China.

Demeton, sold as an amber oily liquid with a sulphur like odour under the name Systox™, is an organophosphate derivative causing irritability and shortness of breath to individuals repeatedly exposed. It was used as a phosphorothioate insecticide and acaricide and has the chemical formula C8H19O3PS2. Although it was previously used as an insecticide, it is now largely obsolete due to its relatively high toxicity to humans. Demeton consists of two components, demeton-S and demeton-O in a ratio of approximately 2:1 respectively. The chemical structure of demeton is closely related to military nerve agents such as VX and a derivative with one of the ethoxy groups replaced by methyl was investigated by both the US and Soviet chemical-weapons programs under the names V.sub.X and GD-7.

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.

Sulfotep (also known as tetraethyldithiopyrophosphate and TEDP) is a pesticide commonly used in greenhouses as a fumigant. The substance is also known as Dithione, Dithiophos, and many other names. Sulfotep has the molecular formula C8H20O5P2S2 and belongs to the organophosphate class of chemicals. It has a cholinergic effect, involving depression of the cholinesterase activity of the peripheral and central nervous system of insects. The transduction of signals is disturbed at the synapses that make use of acetylcholine. Sulfotep is a mobile oil that is pale yellow-colored and smells like garlic. It is primarily used as an insecticide.

Ethoprophos (or ethoprop) is an organophosphate ester with the formula C8H19O2PS2. It is a clear yellow to colourless liquid that has a characteristic mercaptan-like odour. It is used as an insecticide and nematicide and it is an acetylcholinesterase inhibitor.

Glycidamide is an organic compound with the formula H2NC(O)C2H3O. It is a colorless, oil. Structurally, it contains adjacent amides and epoxide functional groups. It is a bioactive, potentially toxic or even carcinogenic metabolite of acrylonitrile and acrylamide. It is a chiral molecule.

4-Ipomeanol (4-IPO) is a pulmonary pre-toxin isolated from sweet potatoes infected with the fungus Fusarium solani. One of the 4-IPO metabolites is toxic to the lungs, liver and kidney in humans and animals. This metabolite can covalently bind to proteins, thereby interfering with normal cell processes.

Nivalenol (NIV) is a mycotoxin of the trichothecene group. In nature it is mainly found in fungi of the Fusarium species. The Fusarium species belongs to the most prevalent mycotoxin producing fungi in the temperate regions of the northern hemisphere, therefore making them a considerable risk for the food crop production industry.

Penicillin Roquefort Toxin is a mycotoxin produced by the fungi Penicillium roqueforti. In 1973, PR toxin was first partially characterized by isolating moldy corn on which the fungi had grown. Although its lethal dose was determined shortly after the isolation of the chemical, details of its toxic effects, were not fully clarified until 1982 in a study with mice, rats, anesthetized cats and preparations of isolated rat auricle.






















