
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases.

A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologically important, and some are both physiologically and commercially significant. A well known dipeptide is aspartame, an artificial sweetener.
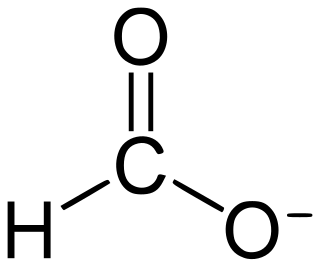
Formate is the conjugate base of formic acid. Formate is an anion or its derivatives such as ester of formic acid. The salts and esters are generally colorless.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
In chemistry, solvolysis is a type of nucleophilic substitution (SN1/SN2) or elimination where the nucleophile is a solvent molecule. Characteristic of SN1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the stereochemical course is complicated by intimate ion pairs, whereby the leaving anion remains close to the carbocation, effectively shielding it from an attack by the nucleophile. Particularly fast reactions can occur by neighbour group participation, with nonclassical ions as intermediates or transition states.
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemicals Ltd, which is more economical and environmentally friendly.
The Criegee rearrangement is a rearrangement reaction named after Rudolf Criegee.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 CFR 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
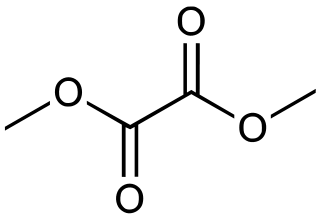
Dimethyl oxalate is an organic compound with the formula (CO2CH3)2 or (CH3)2C2O4. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.
Oleochemistry is the study of vegetable oils and animal oils and fats, and oleochemicals derived from these fats and oils. The resulting product can be called oleochemicals (from Latin: oleum "olive oil"). The major product of this industry is soap, approximately 8.9×6 tons of which were produced in 1990. Other major oleochemicals include fatty acids, fatty acid methyl esters, fatty alcohols and fatty amines. Glycerol is a side product of all of these processes. Intermediate chemical substances produced from these basic oleochemical substances include alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products.

β-Alanine ethyl ester is the ethyl ester of the non-essential amino acid β-alanine. It would be expected to hydrolyse within the body to form β-alanine.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
The Schöllkopf method or Schöllkopf Bis-Lactim Amino Acid Synthesis is a method in organic chemistry for the asymmetric synthesis of chiral amino acids. The method was established in 1981 by Ulrich Schöllkopf. In it glycine is a substrate, valine a chiral auxiliary and the reaction taking place an alkylation.

Hagemann's ester, ethyl 2-methyl-4-oxo-2-cyclohexenecarboxylate, is an organic compound that was first prepared and described in 1893 by German chemist Carl Hagemann. The compound is used in organic chemistry as a reagent in the synthesis of many natural products including sterols, trisporic acids, and terpenoids.
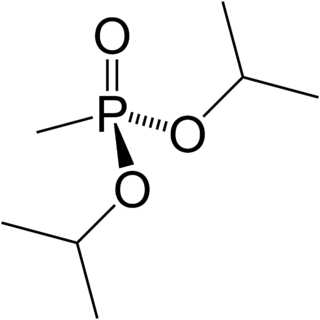
Diisopropyl methylphosphonate (DIMP), also known as diisopropyl methane-phosphonate and phosphonic acid and methyl-bis-(1-methylethyl)ester, is a chemical by-product in the production of sarin gas when two equivalents of isopropyl alcohol react with methylphosphonyl difluoride instead of one.

Allyl iodide (3-iodopropene) is an organic halide used in synthesis of other organic compounds such as N-alkyl-2-pyrrolidones, sorbic acid esters, 5,5-disubstituted barbituric acids, and organometallic catalysts. Allyl iodide can be synthesized from allyl alcohol and methyl iodide on triphenyl phosphite, Finkelstein reaction on allyl halides, or by the action of elemental phosphorus and iodine on glycerol. Allyl iodide dissolved in hexane can be stored for up to three months in a dark freezer at −5 °C (23 °F) before decomposition into free iodine becomes apparent.












