In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Ethanolamine is an organic chemical compound with the formula HOCH
2CH
2NH
2 or C
2H
7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia. ETA molecules are a component in the formation of cellular membranes and are thus a molecular building block for life. It was thought to exist only on Earth and on certain asteroids, but in 2021 evidence was found that ETA molecules exist in interstellar space.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen, carbon dioxide, methane, and water vapour —from coal and water, air and/or oxygen.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from gases. It is a common unit process used in refineries, and is also used in petrochemical plants, natural gas processing plants and other industries.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced at power plants. Its composition depends on what is being burned, but it will usually consist of mostly nitrogen derived from the combustion of air, carbon dioxide, and water vapor as well as excess oxygen. It further contains a small percentage of a number of pollutants, such as particulate matter, carbon monoxide, nitrogen oxides, and sulfur oxides.
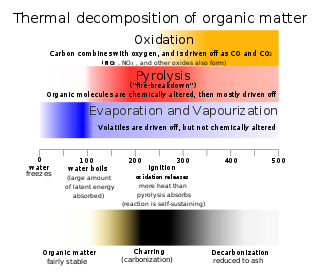
Thermal decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction.

Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" (Group 2B).

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
In electrochemistry, electrosynthesis is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reactions, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.

A thermal oxidizer is a process unit for air pollution control in many chemical plants that decomposes hazardous gases at a high temperature and releases them into the atmosphere.
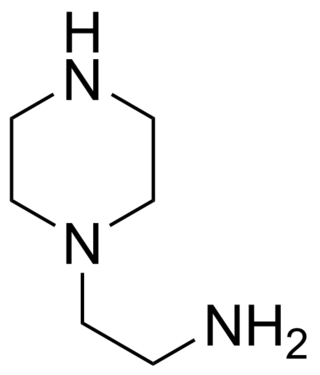
Aminoethylpiperazine (AEP) is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive organic liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. It is REACH and TSCA registered.
A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.
Glycol dehydration is a liquid desiccant system for the removal of water from natural gas and natural gas liquids (NGL). It is the most common and economical means of water removal from these streams. Glycols typically seen in industry include triethylene glycol (TEG), diethylene glycol (DEG), ethylene glycol (MEG), and tetraethylene glycol (TREG). TEG is the most commonly used glycol in industry.
A biogas upgrader is a facility that is used to concentrate the methane in biogas to natural gas standards. The system removes carbon dioxide, hydrogen sulphide, water and contaminants from the biogas. One technique for doing this uses amine gas treating. This purified biogas is also called biomethane. It can be used interchangeably with natural gas.

Boundary Dam Power Station is the largest coal fired station owned by SaskPower, located near Estevan, Saskatchewan, Canada.
Calcium looping (CaL), or the regenerative calcium cycle (RCC), is a second-generation carbon capture technology. It is the most developed form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). The captured carbon dioxide can then be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock. Calcium oxide is often referred to as the sorbent.
The use of ionic liquids in carbon capture is a potential application of ionic liquids as absorbents for use in carbon capture and sequestration. Ionic liquids, which are salts that exist as liquids near room temperature, are polar, nonvolatile materials that have been considered for many applications. The urgency of climate change has spurred research into their use in energy-related applications such as carbon capture and storage.













