
In vitro studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from in vitro experiments may not fully or accurately predict the effects on a whole organism. In contrast to in vitro experiments, in vivo studies are those conducted in living organisms, including humans, known as clinical trials, and whole plants.

Cypermethrin (CP) is a synthetic pyrethroid used as an insecticide in large-scale commercial agricultural applications as well as in consumer products for domestic purposes. It behaves as a fast-acting neurotoxin in insects. It is easily degraded on soil and plants but can be effective for weeks when applied to indoor inert surfaces. Exposure to sunlight, water and oxygen will accelerate its decomposition. Cypermethrin is highly toxic to fish, bees and aquatic insects, according to the National Pesticides Telecommunications Network (NPTN). It is found in many household ant and cockroach killers, including Raid, Ortho, Combat, ant chalk, and some products of Baygon in Southeast Asia.

Phthalates, or phthalate esters, are esters of phthalic acid. They are mainly used as plasticizers, i.e., substances added to plastics to increase their flexibility, transparency, durability, and longevity. They are used primarily to soften polyvinyl chloride (PVC). Note that while phthalates are usually plasticizers, not all plasticizers are phthalates. The two terms are specific and unique and cannot be used interchangeably.

Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine systems. These disruptions can cause numerous adverse human health outcomes including, alterations in sperm quality and fertility, abnormalities in sex organs, endometriosis, early puberty, altered nervous system function, immune function, certain cancers, respiratory problems, metabolic issues, diabetes, obesity, cardiovascular problems, growth, neurological and learning disabilities, and more. Found in many household and industrial products, endocrine disruptors "interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for development, behavior, fertility, and maintenance of homeostasis ."

Anogenital distance (AGD) is the distance from the midpoint of the anus to the genitalia, the underside of the vagina, the clitoris or the scrotum. It is considered medically significant for a number of reasons, in both humans and other animals, including sex determination and as a marker of endocrine disruptor exposure. It is regulated by dihydrotestosterone, which can be disrupted by phthalates common in plastics.
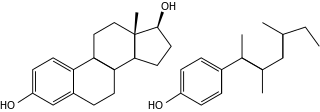
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.

Bis(2-ethylhexyl) phthalate (di-2-ethylhexyl phthalate, diethylhexyl phthalate, diisooctyl phthalate, DEHP; incorrectly — dioctyl phthalate, DIOP) is an organic compound with the formula C6H4(CO2C8H17)2. DEHP is the most common member of the class of phthalates, which are used as plasticizers. It is the diester of phthalic acid and the branched-chain 2-ethylhexanol. This colorless viscous liquid is soluble in oil, but not in water.

Benzyl butyl phthalate (BBP) is an organic compound historically used a plasticizer, but which has now been largely phased out due to health concerns. It is a phthalate ester of containing benzyl alcohol, and n-butanol tail groups. Like most phthalates, BBP is non-volatile and remains liquid over a wide range of temperatures. It was mostly used as a plasticizer for PVC, but was also a common plasticizer for PVCA and PVB.

2-Ethylhexanol (abbreviated 2-EH) is an organic compound with formula C8H18O. It is a branched, eight-carbon chiral alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is produced on a large scale (>2,000,000,000 kg/y) for use in numerous applications such as solvents, flavors, and fragrances and especially as a precursor for production of other chemicals such as emollients and plasticizers. It is encountered in plants, fruits, and wines. The odor has been reported as "heavy, earthy, and slightly floral" for the R enantiomer and "a light, sweet floral fragrance" for the S enantiomer.
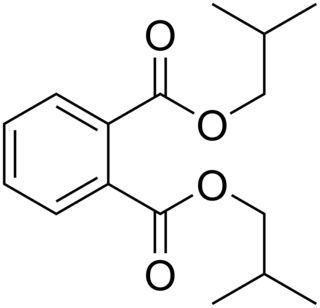
Diisobutyl phthalate (DIBP) is a phthalate ester having the structural formula C6H4(COOCH2CH 2)2. It is formed by the esterification of isobutanol and phthalic anhydride. This and other phthalates are used as plasticizers due to their flexibility and durability. They are found in many industrial and personal products, such as lacquers, nail polish and cosmetics. DIBP can be absorbed via oral ingestion and dermal exposure. When it comes to excretion, DIBP is first converted into the hydrolytic monoester monoisobutyl phthalate (MIBP). The primary excretory route is urine, with biliary excretion being noted in minor amounts. DIBP has lower density and freezing point than the related compound dibutyl phthalate (DBP).

Dimethyl phthalate (DMP) is an organic compound and phthalate ester. it is a colourless and oily liquid that is soluble in organic solvents, but which is only poorly soluble in water.

Diethyl phthalate (DEP) is a phthalate ester. It occurs as a colourless liquid without significant odour but has a bitter, disagreeable taste. It is more dense than water and insoluble in water; hence, it sinks in water.
Bis(2-ethylhexyl) terephthalate commonly abbreviated DEHT (Dioctyl terephthalate or DOTP), is an organic compound with the formula C6H4(CO2C8H17)2. It is a non-phthalate plasticizer, being the diester of terephthalic acid and the branched-chain 2-ethylhexanol, which is often generically referred to as octyl. This colorless viscous liquid is used for softening PVC plastics and is known for chemical similarity to general purpose phthalates such as DEHP and DINP, but without any negative regulatory pressure. It possesses very good plasticizing properties and may be used as a direct replacement for DEHP and DINP in many applications.

Tris(1,3-dichloroisopropyl)phosphate (TDCPP) is a chlorinated organophosphate. Organophosphate chemicals have a wide variety of applications and are used as flame retardants, pesticides, plasticizers, and nerve gases. TDCPP is structurally similar to several other organophosphate flame retardants, such as tris(2-chloroethyl) phosphate (TCEP) and tris(chloropropyl)phosphate (TCPP). TDCPP and these other chlorinated organophosphate flame retardants are all sometimes referred to as "chlorinated tris".
Joseph F. Holson, an American scientist, business executive, and educator in the disciplines of toxicology and product development, served as President of WIL Research Laboratories for 20 years (1988-2008). He is known for his contributions to the fields of developmental and reproductive toxicology (DART), pharmacokinetics, and risk assessment, including extensive experience with study design, data interpretation, and interspecies extrapolation of health effects data. He has served in numerous U.S. EPA/FDA advisory committees and as an expert toxicology witness. He was elected to two National Academy of Sciences toxicology committees. Dr. Holson is an editor and author of the textbook Regulatory Toxicology and an author of two significant chapters in the textbook Developmental and Reproductive Toxicology: A Practical Approach, Second Edition. Two of his peer-reviewed articles were recognized by the Risk Assessment Specialty Section of the Society of Toxicology as the Outstanding Published Papers Demonstrating an Application of Risk Assessment. He is the first author to receive this award in consecutive years for publications produced with two separate sets of coauthors.
Antiandrogens in the environment have become a topic of concern. Many industrial chemicals, including phthalates and pesticides, exhibit antiandrogen activity in animal experiments. Certain plant species have also been found to produce antiandrogens. In animal studies, environmental antiandrogens can harm reproductive organ development in fetuses exposed in utero as well as their offspring.

Monobutyl phthalate (MBP) is an organic compound with the condensed structural formula CH3(CH2)3OOCC6H4COOH. It is a white solid that features both an butyl ester group and a carboxylic acid group. It is the major metabolite of dibutyl phthalate. Like many phthalates, MBP has attracted attention as a potential endocrine disruptor.

Bis(2-ethylhexyl)tetrabromophthalate (or TBPH), is a brominated phthalate derivative with the formula C24H34Br4O4 commonly used as a brominated flame retardant (BFR).

















