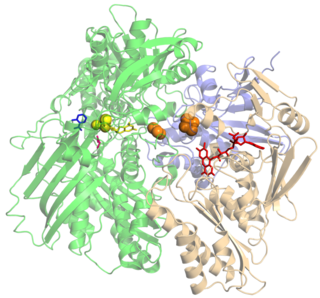
Xanthine oxidase is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.

The glucose oxidase enzyme also known as notatin is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present.
In enzymology, an acyl-CoA oxidase (EC 1.3.3.6) is an enzyme that catalyzes the chemical reaction
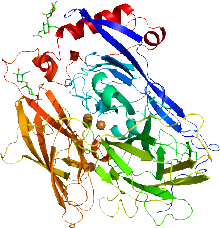
In enzymology, a bilirubin oxidase, BOD or BOx, (EC 1.3.3.5) is an enzyme encoded by a gene in various organisms that catalyzes the chemical reaction
In enzymology, a coproporphyrinogen dehydrogenase (EC 1.3.99.22) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-galactonolactone oxidase (EC 1.3.3.12) is an enzyme that catalyzes the chemical reaction
(S)-Tetrahydroberberine oxidase is an enzyme that catalyzes the final transformation in the biosynthesis of berberine, a quaternary benzylisoquinoline alkaloid of the protoberberine structural subgroup. This reaction pathway catalyzes the four-electron oxidation of (S)-tetrahydroberberine in the presence of oxygen to produce berberine and hydrogen peroxide as products.
In enzymology, a tryptophan alpha,beta-oxidase (EC 1.3.3.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a reticuline oxidase (EC 1.21.3.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a berbamunine synthase (EC 1.14.19.66, Formerly EC 1.1.3.34 and EC 1.14.21.3) is an enzyme that catalyzes the chemical reaction

In enzymology, a cholesterol oxidase (EC 1.1.3.6) is an enzyme that catalyzes the chemical reaction
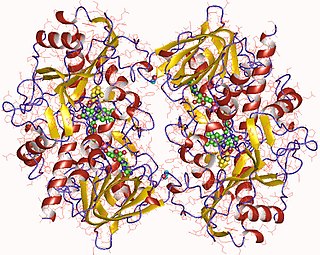
In enzymology, a choline oxidase (EC 1.1.3.17) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acylhexosamine oxidase (EC 1.1.3.29) is an enzyme that catalyzes the chemical reaction
In enzymology, a polyvinyl-alcohol oxidase (EC 1.1.3.30) is an enzyme that catalyzes the chemical reaction
In enzymology, a xylitol oxidase (EC 1.1.3.41) is an enzyme that catalyzes the chemical reaction
In enzymology, an indole 2,3-dioxygenase (EC 1.13.11.17) is an enzyme that catalyzes the chemical reaction
In enzymology, a phenylalanine 2-monooxygenase (EC 1.13.12.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a cyclohexylamine oxidase (EC 1.4.3.12) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutathione oxidase (EC 1.8.3.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-glutamate oxidase (EC 1.4.3.11) is an enzyme that catalyzes the chemical reaction





