Related Research Articles

In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

An antibody (Ab) is the secreted form of a B cell receptor; the term immunoglobulin (Ig) can refer to either the membrane-bound form or the secreted form of the B cell receptor, but they are, broadly speaking, the same protein, and so the terms are often treated as synonymous. Antibodies are large, Y-shaped proteins belonging to the immunoglobulin superfamily which are used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that cause disease. Antibodies can recognize virtually any size antigen with diverse chemical compositions from molecules. Each antibody recognizes one or more specific antigens. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each tip of the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted or inserted into the plasma membrane where they serve as a part of B-cell receptors. When a naïve or memory B cell is activated by an antigen, it proliferates and differentiates into an antibody-secreting effector cell, known as a plasmablast or plasma cell. In addition, B cells present antigens and secrete cytokines. In mammals, including marsupials B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, which is why the B stands for bursa and not bone marrow, as commonly believed.
Humoral immunity is the aspect of immunity that is mediated by macromolecules – including secreted antibodies, complement proteins, and certain antimicrobial peptides – located in extracellular fluids. Humoral immunity is named so because it involves substances found in the humors, or body fluids. It contrasts with cell-mediated immunity. Humoral immunity is also referred to as antibody-mediated immunity.

An automated analyser is a medical laboratory instrument designed to measure various substances and other characteristics in a number of biological samples quickly, with minimal human assistance. These measured properties of blood and other fluids may be useful in the diagnosis of disease.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection, against other foreign proteins, or to one's own proteins. In either case, the procedure is simple.

A radioimmunoassay (RIA) is an immunoassay that uses radiolabeled molecules in a stepwise formation of immune complexes. A RIA is a very sensitive in vitro assay technique used to measure concentrations of substances, usually measuring antigen concentrations by use of antibodies.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
The direct and indirect Coombs tests, also known as antiglobulin test (AGT), are blood tests used in immunohematology. The direct Coombs test detects antibodies that are stuck to the surface of the red blood cells. Since these antibodies sometimes destroy red blood cells they can cause anemia; this test can help clarify the condition. The indirect Coombs test detects antibodies that are floating freely in the blood. These antibodies could act against certain red blood cells; the test can be carried out to diagnose reactions to a blood transfusion.
Cross-matching or crossmatching is a test performed before a blood transfusion as part of blood compatibility testing. Normally, this involves adding the recipient's blood plasma to a sample of the donor's red blood cells. If the blood is incompatible, the antibodies in the recipient's plasma will bind to antigens on the donor red blood cells. This antibody-antigen reaction can be detected through visible clumping or destruction of the red blood cells, or by reaction with anti-human globulin. Along with blood typing of the donor and recipient and screening for unexpected blood group antibodies, cross-matching is one of a series of steps in pre-transfusion testing. In some circumstances, an electronic cross-match can be performed by comparing records of the recipient's ABO and Rh blood type against that of the donor sample. In emergencies, blood may be issued before cross-matching is complete. Cross-matching is also used to determine compatibility between a donor and recipient in organ transplantation.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
A precipitin is an antibody which can precipitate out of a solution upon antigen binding.

The immunoglobulin light chain is the small polypeptide subunit of an antibody (immunoglobulin).
Radial immunodiffusion (RID), Mancini immunodiffusion or single radial immunodiffusion assay, is an immunodiffusion technique used in immunology to determine the quantity or concentration of an antigen in a sample.
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
Turbidimetry is the process of measuring the loss of intensity of transmitted light due to the scattering effect of particles suspended in it. Light is passed through a filter creating a light of known wavelength which is then passed through a cuvette containing a solution. A photoelectric cell collects the light which passes through the cuvette. A measurement is then given for the amount of absorbed light.
Immunoglobulin therapy is the use of a mixture of antibodies to treat several health conditions. These conditions include primary immunodeficiency, immune thrombocytopenic purpura, chronic inflammatory demyelinating polyneuropathy, Kawasaki disease, certain cases of HIV/AIDS and measles, Guillain–Barré syndrome, and certain other infections when a more specific immunoglobulin is not available. Depending on the formulation it can be given by injection into muscle, a vein, or under the skin. The effects last a few weeks.

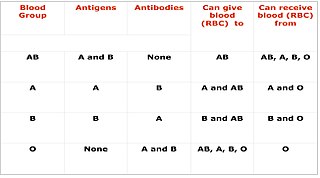
Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.
References
- ↑ MedlinePlus Encyclopedia : 003545
- ↑ Reese, Andy C.; Dolen, William K. (1998). "Chapter 4: Antigen-Antibody Reactions, Nephelometry". Essentials of Immunology. Medical College of Georgia. Archived from the original on September 4, 2006.
- 1 2 "Nephelometry". BMG LabTech. 2022. Retrieved 9 November 2022.
- ↑ Stevens, Christine Dorresteyn (2010). Clinical Immunology and Serology (3rd ed.). F.A. Davis Company. p. 127. ISBN 978-0803618145.
- ↑ Fondriest Environmental, Inc. (5 September 2014). "Measuring Turbidity, TSS, and Water Clarity". Fundamentals of Environmental Measurements. Retrieved 9 November 2022.
- ↑ Thoré, Eli S. J.; Schoeters, Floris; Spit, Jornt; Van Miert, Sabine (September 2021). "Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry". Processes. 9 (9): 1530. doi: 10.3390/pr9091530 .
- ↑ Mechsner, K.L. (1 January 1984). "An automated nephelometric system for evaluation of the growth of bacterial cultures". Analytica Chimica Acta. 163: 85–90. Bibcode:1984AcAC..163...85M. doi:10.1016/S0003-2670(00)81496-8.