Diagnosis
| | This section is empty. You can help by adding to it. (December 2017) |
| Neutrophil-specific granule deciciency | |
|---|---|
| Other names | SGD |
| Specialty | Immunology |
Neutrophil-specific granule deficiency [1] ( previously known as lactoferrin deficiency) is a rare congenital immunodeficiency characterized by an increased risk for pyogenic infections due to defective production of specific granules and gelatinase granules in patient neutrophils.
Atypical infections are the key clinical manifestation of SGD. [1] Within the first few years of life, patients will experience repeated pyogenic infections by species such as Staphylococcus aureus , Pseudomonas aeruginosa or other Enterobacteriaceae, and Candida albicans . Cutaneous ulcers or abscesses and pneumonia and chronic lung disease are common. Patients may also develop sepsis, mastoiditis, otitis media, and lymphadenopathy. Infants may present with vomiting, diarrhea, and failure to thrive. [2]
Diagnosis can be made based upon CEBPE gene mutation or a pathognomonic finding of a blood smear showing lack of specific granules. Neutrophils and eosinophils will contain hyposegmented nuclei (a pseudo-Pelger–Huet anomaly).
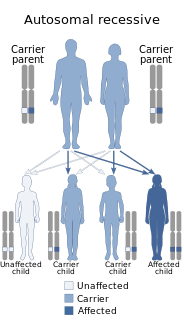
A majority of patients with SGD have been found to have mutations in the CEBPE (CCAAT/enhancer-binding protein epsilon) gene, a transcription factor primarily active in myeloid cells. [3] Almost all patients have been found to be homozygous for the mutation, suggesting the disease is autosomal recessive. One patient, heterozygous for the mutation, was found to be deficient in GFI1, a related gene. [4]
The defect in CEBPE appears to block the ability of neutrophils to mature past the promyelocyte stage in bone marrow. [3] Since specific (secondary) and gelatinase (tertiary) granules are only produced past the promyelocyte stage of development, these are deficient in SGD. Lactoferrin is the major enzyme found in specific granules, and will be largely absent in the granulocytes of these patients, along with defensins (despite these also being found in azurophilic (primary) granules). [5] The other major components of azurophilic granules, such as lysozyme, cathepsin, and elastase will be normal, however a lack of defensins and lactoferrin drastically weakens the neutrophil innate ability to fight infection. Neutrophils will also display abnormal chemotaxis, such as a decreased response to fMLP, due to a lack of chemotactic receptors typically found in the specific granules. [6]
| | This section is empty. You can help by adding to it. (December 2017) |
Treatment consists mainly of high dose antibiotics for active infections and prophylactic antibiotics for prevention of future infections. GM-CSF therapy or bone marrow transplant might be considered for severe cases. [2] Prognosis is difficult to predict, but patients receiving treatment are generally able to survive to adulthood.[ citation needed ]
Estimation of the frequency of SGD is difficult, as it is an extremely rare disease with few cases reported in literature. The condition was first reported in 1980, and since only a handful more cases have been published.

Granulocytes are cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. They are also called polymorphonuclear leukocytes because of the varying shape of the nucleus, which is usually lobed into three segments. This distinguishes them from the mononuclear agranulocytes. The term polymorphonuclear leukocyte often refers specifically to "neutrophil granulocytes", the most abundant of the granulocytes; the other types have fewer lobes. Granulocytes are produced via granulopoiesis in the bone marrow.
Immunodeficiency, also known as immunocompromisation, is a state in which the immune system's ability to fight infectious diseases and cancer is compromised or entirely absent. Most cases are acquired ("secondary") due to extrinsic factors that affect the patient's immune system. Examples of these extrinsic factors include HIV infection and environmental factors, such as nutrition. Immunocompromisation may also be due to genetic diseases/flaws such as SCID.

Chronic granulomatous disease (CGD), also known as Bridges–Good syndrome, chronic granulomatous disorder, and Quie syndrome, is a diverse group of hereditary diseases in which certain cells of the immune system have difficulty forming the reactive oxygen compounds used to kill certain ingested pathogens. This leads to the formation of granulomas in many organs. CGD affects about 1 in 200,000 people in the United States, with about 20 new cases diagnosed each year.

Myeloperoxidase deficiency is an autosomal recessive genetic disorder featuring deficiency, either in quantity or of function, of myeloperoxidase, a peroxidase enzyme expressed by neutrophil granulocytes. It is classified as a primary immunodeficiency disorder, and is caused by a mutation in the myeloperoxidase gene on chromosome 17q23. Between 1:1000 and 1:4000 of people in the United States and Europe are myeloperoxidase-deficient. It can appear similar to chronic granulomatous disease on some screening tests.
Myeloperoxidase (MPO) is a peroxidase enzyme that in humans is encoded by the MPO gene on chromosome 17. MPO is most abundantly expressed in neutrophil granulocytes, and produces hypohalous acids to carry out their antimicrobial activity, including hypochlorous acid, the sodium salt of which is the chemical in bleach. It is a lysosomal protein stored in azurophilic granules of the neutrophil and released into the extracellular space during degranulation. Neutrophil myeloperoxidase has a heme pigment, which causes its green color in secretions rich in neutrophils, such as mucus and sputum. The green color contributed to its outdated name verdoperoxidase.
Cyclic neutropenia (CyN) is a rare hematologic disorder and form of congenital neutropenia that tends to occur approximately every three weeks and lasting for few days at a time due to changing rates of neutrophil production by the bone marrow. It causes a temporary condition with a low absolute neutrophil count and because the neutrophils make up the majority of circulating white blood cells it places the body at severe risk of inflammation and infection. In comparison to severe congenital neutropenia, it responds well to treatment with granulocyte colony-stimulating factor (filgrastim), which increases the neutrophil count, shortens the cycle length, as well decreases the severity and frequency of infections.
Béguez-Chédiak–Higashi syndrome (CHS) is a rare autosomal recessive disorder that arises from a mutation of a lysosomal trafficking regulator protein, which leads to a decrease in phagocytosis. The decrease in phagocytosis results in recurrent pyogenic infections, albinism, and peripheral neuropathy.
Severe congenital neutropenia (SCN), also often known as Kostmann syndrome or disease, is a group of rare disorders that affect myelopoiesis, causing a congenital form of neutropenia, usually without other physical malformations. SCN manifests in infancy with life-threatening bacterial infections.
Leukocyte adhesion deficiency (LAD), is a rare autosomal recessive disorder characterized by immunodeficiency resulting in recurrent infections. LAD is currently divided into three subtypes: LAD1, LAD2, and the recently described LAD3, also known as LAD-1/variant. In LAD3, the immune defects are supplemented by a Glanzmann thrombasthenia-like bleeding tendency.
Specific granules are secretory vesicles found exclusively in cells of the immune system called granulocytes.

Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally been thought to kill invading pathogens through two strategies: engulfment of microbes and secretion of anti-microbials. In 2004, a novel third function was identified: formation of NETs. NETs allow neutrophils to kill extracellular pathogens while minimizing damage to the host cells. Upon in vitro activation with the pharmacological agent phorbol myristate acetate (PMA), Interleukin 8 (IL-8) or lipopolysaccharide (LPS), neutrophils release granule proteins and chromatin to form an extracellular fibril matrix known as NET through an active process.

Lipocalin-2 (LCN2), also known as oncogene 24p3 or neutrophil gelatinase-associated lipocalin (NGAL), is a protein that in humans is encoded by the LCN2 gene. NGAL is involved in innate immunity by sequestering iron and preventing its use by bacteria, thus limiting their growth. It is expressed in neutrophils and in low levels in the kidney, prostate, and epithelia of the respiratory and alimentary tracts. NGAL is used as a biomarker of kidney injury.

Alpha defensins are a family of mammalian defensin peptides of the alpha subfamily. In mammals they are also known as cryptdins and are produced within the small bowel. Cryptdin is a portmanteau of crypt and defensin.

Defensin, alpha 1 also known as human alpha defensin 1, human neutrophil peptide 1 (HNP-1) or neutrophil defensin 1 is a human protein that is encoded by the DEFA1 gene. Human alpha defensin 1 belongs to the alpha defensin family of antimicrobial peptides.

CCAAT/enhancer binding protein (C/EBP), epsilon, also known as CEBPE and CRP1, is a type of ccaat-enhancer-binding protein. CEBPE is its human gene and is pro-apoptotic.

Zinc finger protein Gfi-1 is a transcriptional repressor that in humans is encoded by the GFI1 gene. It is important normal hematopoiesis.
JAK3 deficiency is a dysfunction in cytokine receptor signalling and their production of cytokines.
Defensin, alpha 4 (DEFA4), also known as neutrophil defensin 4 or HNP4, is a human defensin peptide that is encoded by the DEFA4 gene. HNP4 is expressed in the granules of the neutrophil where it defends the host against bacteria and viruses.

Eosinophil peroxidase is an enzyme found within the eosinophil granulocytes, innate immune cells of humans and mammals. This oxidoreductase protein is encoded by the gene EPX, expressed within these myeloid cells. EPO shares many similarities with its orthologous peroxidases, myeloperoxidase (MPO), lactoperoxidase (LPO), and thyroid peroxidase (TPO). The protein is concentrated in secretory granules within eosinophils. Eosinophil peroxidase is a heme peroxidase, its activities including the oxidation of halide ions to bacteriocidal reactive oxygen species, the cationic disruption of bacterial cell walls, and the post-translational modification of protein amino acid residues.
An innate immune defect is a defect in the innate immune response that blunts the response to infection. These defects may occur in monocytes, neutrophils, natural killer cells, basophils, mast cells or complement proteins.
| Classification |
|---|