| |
| Names | |
|---|---|
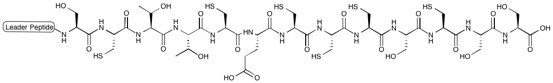
| IUPAC name N-(3-Amino-3-oxoprop-1-en-2-yl)-2-[(21Z)-21-ethylidene-9,30-dihydroxy-18-(1-hydroxyethyl)-40-methyl-16,19,26,31,42,46-hexaoxo-32-oxa-3,13,23,43,49-pentathia-7,17,20,27,45,51,52,53,54,55-decazanonacyclo[26.16.6.12,5.112,15.122,25.138,41.147,50.06,11.034,39]pentapentaconta-2(55),4,6,8,10,12(54),14,22(53),24,34(39),35,37,40,47,50-pentadecaen-8-yl]-1,3-thiazole-4-carboxamide | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.054.654 |
| EC Number |
|
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C51H43N13O12S6 | |
| Molar mass | 1222.34 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Nosiheptide is a thiopeptide antibiotic produced by the bacterium Streptomyces actuosus . [1] [2] [3]