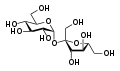
A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula Cm(H2O)n (where m may or may not be different from n). However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. formaldehyde and acetic acid).

A disaccharide is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose.
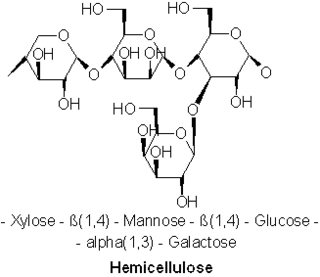
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. While cellulose is crystalline, strong, and resistant to hydrolysis, hemicelluloses have random, amorphous structure with little strength. They are easily hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

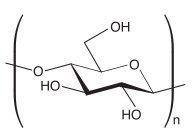
Polysaccharides, or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin.
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.

Maltose, also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When alpha-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar.
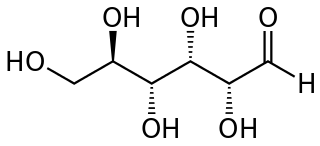
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.
An Endoglycosidase is an enzyme that releases oligosaccharides from glycoproteins or glycolipids. It may also cleave polysaccharide chains between residues that are not the terminal residue, although releasing oligosaccharides from conjugated protein and lipid molecules is more common.
Carbohydrase is the name of a set of enzymes that catalyze 5 types of reactions, turning carbohydrates into simple sugars, from the large family of glycosidases.
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.

Beta-amylase is an enzyme with the systematic name 4-alpha-D-glucan maltohydrolase. This enzyme catalyses the following chemical reaction:
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the detection, synthesis, structure, and function of carbohydrates. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the selective reaction of hydroxyl groups; as a result, it relies heavily on the use of protecting groups.
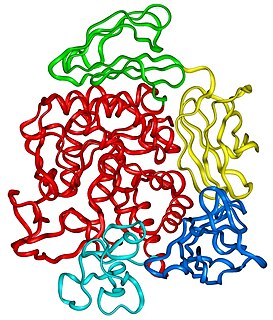
In enzymology, a cyclomaltodextrin glucanotransferase is an enzyme that catalyzes the chemical reaction of cyclizing part of a 1,4-alpha-D-glucan molecule through the formation of a 1,4-alpha-D-glucosidic bond. They are bacterial enzymes belonging to the same family of the α-amylase specifically known as glycosyl-hydrolase family 13. This peculiar enzyme is capable of catalyzing more than one reaction with the most important being the synthesis of non-reducing cyclic dextrins known as cyclodextrins starting from starch, amylose, and other polysaccharides.
Carbohydrate conformation refers to the overall three-dimensional structure adopted by a carbohydrate (saccharide) molecule as a result of the through-bond and through-space physical forces it experiences arising from its molecular structure. The physical forces that dictate the three-dimensional shapes of all molecules—here, of all monosaccharide, oligosaccharide, and polysaccharide molecules—are sometimes summarily captured by such terms as "steric interactions" and "stereoelectronic effects".
Monosaccharide nomenclature is the naming system of the building blocks of carbohydrates, the monosaccharides, which may be monomers or part of a larger polymer. Monosaccharides are subunits that cannot be further hydrolysed in to simpler units. Depending on the number of carbon atom they are further classified into trioses, tetroses, pentoses, hexoses etc., which is further classified in to aldoses and ketoses depending on the type of functional group present in them.
Carbohydrate NMR Spectroscopy is the application of nuclear magnetic resonance (NMR) spectroscopy to structural and conformational analysis of carbohydrates. This method allows the scientists to elucidate structure of monosaccharides, oligosaccharides, polysaccharides, glycoconjugates and other carbohydrate derivatives from synthetic and natural sources. Among structural properties that could be determined by NMR are primary structure, saccharide conformation, stoichiometry of substituents, and ratio of individual saccharides in a mixture. Modern high field NMR instruments used for carbohydrate samples, typically 500 MHz or higher, are able to run a suite of 1D, 2D, and 3D experiments to determine a structure of carbohydrate compounds.
Carbohydrate synthesis is a sub-field of organic chemistry concerned specifically with the generation of natural and unnatural carbohydrate structures. This can include the synthesis of monosaccharide residues or structures containing more than one monosaccharide, known as oligosaccharides.

Glucansucrase is an enzyme in the glycoside hydrolase family GH70 used by lactic acid bacteria to split sucrose and use resulting glucose molecules to build long, sticky biofilm chains. These extracellular homopolysaccharides are called α-glucan polymers.

Glucanases are enzymes that break down large polysaccharides via hydrolysis. The product of the hydrolysis reaction is called a glucan, a linear polysaccharide made of up to 1200 glucose monomers, held together with glycosidic bonds. Glucans are abundant in the endosperm cell walls of cereals such as barley, rye, sorghum, rice, and wheat. Glucanases are also referred to as lichenases, hydrolases, glycosidases, glycosyl hydrolases, and/or laminarinases. Many types of glucanases share similar amino acid sequences but vastly different substrates. Of the known endo-glucanases, 1,3-1,4-β-glucanase is considered the most active.
Isomaltooligosaccharide (IMO) is a mixture of short-chain carbohydrates which has a digestion-resistant property. IMO is found naturally in some foods, as well as being manufactured commercially. The raw material used for manufacturing IMO is starch, which is enzymatically converted into a mixture of isomaltooligosaccharides.

![Allyl a-L-fucopyranosyl-(1-3)-[a-D-galactopyranosyl-(1-4)]-a-D-glucopyranosyl-(1-3)-a-D-galactopyranoside Branched oligosaccharide struct.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/a/a0/Branched_oligosaccharide_struct.svg/480px-Branched_oligosaccharide_struct.svg.png)