 | |
 | |
| Names | |
|---|---|
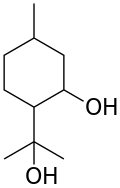
| Preferred IUPAC name 2-(2-Hydroxypropan-2-yl)-5-methylcyclohexan-1-ol | |
| Other names 2-(1-Hydroxy-1-methylethyl)-5-methylcyclohexanol para-Menthane-3,8-diol 2-Hydroxy-α,α,4-trimethylcyclohexanemethanol | |
| Identifiers | |
3D model (JSmol) | |
| 2552262 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.050.849 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H20O2 | |
| Molar mass | 172.268 g·mol−1 |
| Density | 1.009 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
p-Menthane-3,8-diol, also known as para-menthane-3,8-diol, PMD, or menthoglycol, is an organic compound classified as a diol and a terpenoid. It is colorless. Its name reflects the hydrocarbon backbone, which is that of p-menthane. A total of eight stereoisomers are possible, based on the three stereocenters of the ring. Depending on the source, one or more may predominate.
Contents
PMD is the active ingredient in some insect repellents. Its odor and chemical structure are similar to menthol, and it has a cooling feel. [1] It is found in small quantities in the essential oil from the leaves of Corymbia citriodora , formerly known as Eucalyptus citriodora. This tree is native to Australia, but is now cultivated in many warm places around the world. C. citriodora oil, when refined to increase its PMD content for use in insect repellents, is known in the United States as oil of lemon eucalyptus (OLE) as well as Citriodiol. [2] Citriodora oil contains only 1–2% PMD, while refined OLE contains approximately up to 70% PMD. [3] Some commercial PMD products are not made from C. citriodora oil, but rather from synthetic citronellal.