
A pandemic is an epidemic of an infectious disease that has spread across a large region, for instance multiple continents or worldwide, affecting a substantial number of individuals. Widespread endemic diseases with a stable number of infected individuals such as recurrences of seasonal influenza are generally excluded as they occur simultaneously in large regions of the globe rather than being spread worldwide.
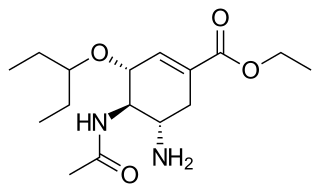
Oseltamivir, sold under the brand name Tamiflu, is an antiviral medication used to treat and prevent influenza A and influenza B, viruses that cause the flu. Many medical organizations recommend it in people who have complications or are at high risk of complications within 48 hours of first symptoms of infection. They recommend it to prevent infection in those at high risk, but not the general population. The Centers for Disease Control and Prevention (CDC) recommends that clinicians use their discretion to treat those at lower risk who present within 48 hours of first symptoms of infection. It is taken by mouth, either as a pill or liquid.

Influenza vaccines, colloquially known as flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. Vaccination against influenza began in the 1930s, with large-scale availability in the United States beginning in 1945.

Swine influenza is an infection caused by any of several types of swine influenza viruses. Swine influenza virus (SIV) or swine-origin influenza virus (S-OIV) refers to any strain of the influenza family of viruses that is endemic in pigs. As of 2009, identified SIV strains include influenza C and the subtypes of influenza A known as H1N1, H1N2, H2N1, H3N1, H3N2, and H2N3.

In virology, influenza A virus subtype H1N1 (A/H1N1) is a subtype of influenza A virus. Major outbreaks of H1N1 strains in humans include the 1918 Spanish flu pandemic, the 1977 Russian flu pandemic and the 2009 swine flu pandemic. It is an orthomyxovirus that contains the glycoproteins hemagglutinin (H) and neuraminidase (N), antigens whose subtypes are used to classify the strains of the virus as H1N1, H1N2 etc. Hemagglutinin causes red blood cells to clump together and binds the virus to the infected cell. Neuraminidase is a type of glycoside hydrolase enzyme which helps to move the virus particles through the infected cell and assist in budding from the host cells.

An influenza pandemic is an epidemic of an influenza virus that spreads across a large region and infects a large proportion of the population. There have been six major influenza epidemics in the last 140 years, with the 1918 flu pandemic being the most severe; this is estimated to have been responsible for the deaths of 50–100 million people. The 2009 swine flu pandemic resulted in under 300,000 deaths and is considered relatively mild. These pandemics occur irregularly.

Influenza A virus subtype H3N2 (A/H3N2) is a subtype of viruses that causes influenza (flu). H3N2 viruses can infect birds and mammals. In birds, humans, and pigs, the virus has mutated into many strains. In years in which H3N2 is the predominant strain, there are more hospitalizations.

Human mortality from H5N1 or the human fatality ratio from H5N1 or the case-fatality rate of H5N1 is the ratio of the number of confirmed human deaths resulting from confirmed cases of transmission and infection of H5N1 to the number of those confirmed cases. For example, if there are 100 confirmed cases of humans infected with H5N1 and 50 die, then there is a 50% human fatality ratio. H5N1 flu is a concern due to the global spread of H5N1 that constitutes a pandemic threat. The majority of H5N1 flu cases have been reported in southeast and east Asia. The case-fatality rate is central to pandemic planning. Estimates of case-fatality (CF) rates for past influenza pandemics have ranged from to 2-3% for the 1918 pandemic to about 0.6% for the 1957 pandemic to 0.2% for the 1968 pandemic. As of 2008, the official World Health Organization estimate for the case-fatality rate for the outbreak of H5N1 avian influenza was approximately 60%. Public health officials in Ontario, Canada argue that the true case-fatality rate could be lower, pointing to studies suggesting it could be 14-33%, and warned that it was unlikely to be as low as the 0.1–0.4% rate that was built into many pandemic plans.

The 2009 swine flu pandemic, caused by the H1N1/swine flu/influenza virus and declared by the World Health Organization (WHO) from June 2009 to August 2010, was the third recent flu pandemic involving the H1N1 virus. The first identified human case was in La Gloria, Mexico, a rural town in Veracruz. The virus appeared to be a new strain of H1N1 that resulted from a previous triple reassortment of bird, swine, and human flu viruses which further combined with a Eurasian pig flu virus, leading to the term "swine flu".
The 2009 flu pandemic in the United States was caused by a novel strain of the Influenza A/H1N1 virus, commonly referred to as "swine flu", that was first detected on 15 April 2009. While the 2009 H1N1 virus strain was commonly referred to as "swine flu", there is no evidence that it is endemic to pigs or of transmission from pigs to people; instead, the virus spreads from person to person. On April 25, the World Health Organization declared a public health emergency, followed concurringly by the Obama administration on April 26.

This article covers the chronology of the 2009 novel influenza A (H1N1) pandemic. Flag icons denote the first announcements of confirmed cases by the respective nation-states, their first deaths, and relevant sessions and announcements of the World Health Organization (WHO), the European Union , and the U.S. Centers for Disease Control (CDC).

The pandemic H1N1/09 virus is a swine origin influenza A virus subtype H1N1 strain that was responsible for the 2009 swine flu pandemic. This strain is often called swine flu by the public media due to the prevailing belief that it originated in pigs. The virus is believed to have originated around September 2008 in central Mexico.

The 2009 swine flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed virus was injected, while the live virus was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009.
Influenza prevention involves taking steps that one can use to decrease their chances of contracting flu viruses, such as the Pandemic H1N1/09 virus, responsible for the 2009 flu pandemic.
2009 flu pandemic in Taiwan began on May 20, 2009, when a non-citizen who had been living in Taiwan returned from the United States via Hong Kong. By the end of September, more than 90% of influenza A detected in the community were Influenza A (H1N1).

Influenza A virus subtype H7N9 (A/H7N9) is a bird flu strain of the species Influenza virus A. Avian influenza A H7 viruses normally circulate amongst avian populations with some variants known to occasionally infect humans. An H7N9 virus was first reported to have infected humans in March 2013, in China. Cases continued to be reported throughout April and then dropped to only a few cases during the summer months. At the closing of the year, 144 cases had been reported of which 46 had died. It is known that influenza tends to strike during the winter months, and the second wave, which began in October, was fanned by a surge in poultry production timed for Lunar New Year feasts that began at the end of January. January 2014 brought a spike in reports of illness with 96 confirmed reports of disease and 19 deaths. As of April 11, 2014, the outbreak's overall total was 419, including 7 in Hong Kong, and the unofficial number of deaths was 127.
The Pandemic Severity Assessment Framework (PSAF) is an evaluation framework published by the Centers for Disease Control and Prevention in 2016 which uses quadrants to evaluate both the transmissibility and clinical severity of an influenza pandemic and to combine these into an overall impact estimate. Clinical severity is calculated via multiple measures including case fatality rate, case-hospitalization ratios, and deaths-hospitalizations ratios, while viral transmissibility is measured via available data among secondary household attack rates, school attack rates, workplace attack rates, community attack rates, rates of emergency department and outpatient visits for influenza-like illness.
Planning and preparing for pandemics has happened in countries and international organizations. The World Health Organization writes recommendations and guidelines, though there is no sustained mechanism to review countries' preparedness for epidemics and their rapid response abilities. National action depends on national governments. In 2005–2006, before the 2009 swine flu pandemic and during the decade following it, the governments in the United States, France, UK, and others managed strategic health equipment stocks, but they often reduced stocks after the 2009 pandemic in order to reduce costs.
The Disease Outbreak Response System Condition (DORSCON) is a disease crisis management plan in Singapore. The system is colour-coded reflecting the disease situation in Singapore. Beside showing the disease situation, it also outline the impact on the general public and what the general public should do.
Type A influenza vaccine is for the prevention of infection of influenza A virus and also the influenza-related complications. Different monovalent type A influenza vaccines have been developed for different subtypes of influenza A virus including H1N1 and H5N1. Both intramuscular injection or intranasal spray are available on market. Unlike the seasonal influenza vaccines which are used annually, they are usually used during the outbreak of certain strand of subtypes of influenza A. Common adverse effects includes injection site reaction and local tenderness. Incidences of headache and myalgia were also reported with H1N1 whereas cases of fever has also been demonstrated with H5N1 vaccines. It is stated that immunosuppressant therapies would reduce the therapeutic effects of vaccines and that people with egg allergy should go for the egg-free preparations.










