
Penicillium is a genus of ascomycetous fungi that is part of the mycobiome of many species and is of major importance in the natural environment, in food spoilage, and in food and drug production.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Mevastatin is a hypolipidemic agent that belongs to the statins class.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it causes different toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.

Akira Endo is a Japanese biochemist whose research into the relationship between fungi and cholesterol biosynthesis led to the development of statin drugs, which are some of the best-selling pharmaceuticals in history.
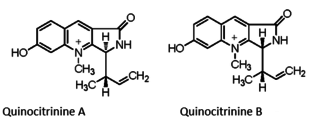
Quinocitrinines are quinoline alkaloids isolated from a permafrost Penicillium.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.
Penicillium brefeldianum is an anamorph fungus species of the genus of Penicillium which produces Brefeldin A a fungal metabolite.
Penicillium chermesinum is an anamorph fungus species of the genus of Penicillium which was isolated from soil from Nova Scotia in Canada.Penicillium chermesinum produces plastatin, luteosporin, xanthomegnin, azaphilones, p-terphenyls and costaclavine.
Penicillium corylophilum is a species of the genus of Penicillium which occurs in damp buildings in United States, Canada and western Europe but it can also be found in a variety of foods and mosquitoes. Penicillium corylophilum produces the alkaloid epoxyagroclavine and citrinin and is a pathogen to mosquitoes.
Penicillium decumbens is an anamorph species of the genus of Penicillium which occurs widespread in nature, mainly in subtropical and tropical soil but it also occur in food. Analysis have shown that Penicillium decumbens has antibiotic activity Penicillium decumbens produces the cyclopentenone cyclopenicillone
Penicillium gorlenkoanum is a species of the genus of Penicillium which produces citrinin, costaclavine and epicostaclavine.
Penicillium herquei is an anamorph, filamentous species of the genus of Penicillium which produces citreorosein, emodin, hualyzin, herquline B, janthinone, citrinin and duclauxin,.
Penicillium hetheringtonii is a species of the genus of Penicillium which is named after A.C. Hetherington. This species was first isolated from beach soil in Land's End Garden in Treasure Island, Florida in the United States. Penicillium hetheringtonii produces citrinin and quinolactacin.
Penicillium janczewskii is an anamorph and filamentous species of the genus of Penicillium which was isolated from the rhizosphere of Vernonia herbacea. Penicillium janczewskii produces griseofulvin
Penicillium oxalicum is an anamorph species of the genus Penicillium which was isolated from rhizosphere soil of pearl millet. Penicillium oxalicum produces secalonic acid D, chitinase, oxalic acid, oxaline and β-N-acetylglucosaminidase and occurs widespread in food and tropical commodities. This fungus could be used against soilborne diseases like downy mildew of tomatoes
Penicillium paxilli is an anamorph, saprophytic species of the genus Penicillium which produces paxilline, paxisterol, penicillone, pyrenocine A, paspaline B and verruculogene. Penicillium paxilli is used as a model to study the biochemistry of the indol-diterepene biosynthesis
Penicillium steckii is a species of fungus in the genus Penicillium which produces citrinin, tanzawaic acid E, tanzawaic acid F.
Penicillium waksmanii is an anamorph species of the genus of Penicillium which was isolated from the alga Sargassum ringgoldianum. Penicillium waksmanii produces pyrenocine A, pyrenocine C, pyrenocine D and pyrenocine E




