
Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group bonded to a hydroxy group. Mildly acidic, it requires careful handling because it can cause chemical burns.

Microcline (KAlSi3O8) is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during slow cooling of orthoclase; it is more stable at lower temperatures than orthoclase. Sanidine is a polymorph of alkali feldspar stable at yet higher temperature. Microcline may be clear, white, pale-yellow, brick-red, or green; it is generally characterized by cross-hatch twinning that forms as a result of the transformation of monoclinic orthoclase into triclinic microcline.

Sodium chloride, commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chlorine ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.

Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic (also called, ring) structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.

Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and rose-like odor that is soluble in organic solvents. Benzophenone is the simplest diaromatic ketone. It is a widely used building block in organic chemistry, being the parent diarylketone.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.

Sodium hexametaphosphate (SHMP) is a salt of composition Na6[(PO3)6]. Sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: NaPO3), of which the hexamer is one, and is usually the compound referred to by this name. Such a mixture is more correctly termed sodium polymetaphosphate. They are white solids that dissolve in water.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.

Liquid smoke is a water-soluble yellow to red liquid used as a flavoring as a substitute for cooking with wood smoke while retaining a similar flavor. It can be used to flavor any meat or vegetable. It is available as pure condensed smoke from various types of wood, and as derivative formulas containing additives.

Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper.
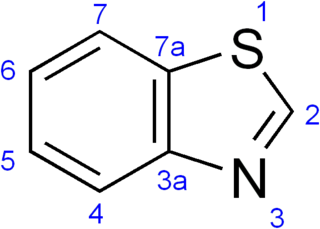
Benzothiazole, or more specifically 1,3-benzothiazole, is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.

Sodium hypophosphite (NaPO2H2, also known as sodium phosphinate) is the sodium salt of hypophosphorous acid and is often encountered as the monohydrate, NaPO2H2·H2O. It is a solid at room temperature, appearing as odorless white crystals. It is soluble in water, and easily absorbs moisture from the air.

Sodium phenoxide (sodium phenolate) is an organic compound with the formula NaOC6H5. It is a white crystalline solid. Its anion, phenoxide, also known as phenolate, is the conjugate base of phenol. It is used as a precursor to many other organic compounds, such as aryl ethers.

Dehydroacetic acid is an organic compound which has several industrial applications. The compound is classified as a pyrone derivative. It presents as an odorless, colorless to white crystalline powder, almost insoluble in water and moderately soluble in most organic solvents.

15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+) and potassium (K+); however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such as a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry.

Phenylsodium C6H5Na is an organosodium compound. Solid phenylsodium was first isolated by Nef in 1903. Although the behavior of phenylsodium and phenyl magnesium bromide are similar, the organosodium compound is very rarely used.
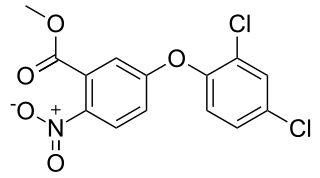
Bifenox is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase which is necessary for chlorophyll synthesis.



















