
Acinetobacter is a genus of Gram-negative bacteria belonging to the wider class of Gammaproteobacteria. Acinetobacter species are oxidase-negative, exhibit twitching motility, and occur in pairs under magnification.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
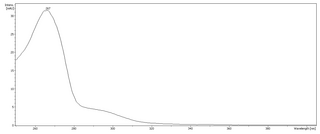
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed polyphenols.

Micrococcus is a genus of bacteria in the Micrococcaceae family. Micrococcus occurs in a wide range of environments, including water, dust, and soil. Micrococci have Gram-positive spherical cells ranging from about 0.5 to 3 micrometers in diameter and typically appear in tetrads. They are catalase positive, oxidase positive, indole negative and citrate negative. Micrococcus has a substantial cell wall, which may comprise as much as 50% of the cell mass. The genome of Micrococcus is rich in guanine and cytosine (GC), typically exhibiting 65 to 75% GC-content. Micrococci often carry plasmids that provide the organism with useful traits.

4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Catechol 1,2- dioxygenase is an enzyme that catalyzes the oxidative ring cleavage of catechol to form cis,cis-muconic acid:
Acinetobacter calcoaceticus is a bacterial species of the genus Acinetobacter. It is a nonmotile, Gram-negative coccobacillus. It grows under aerobic conditions, is catalase positive and oxidase negative. A. calcoaceticus is a part of the A. calcoaceticus-A. baumannii complex together with Acinetobacter baumannii, Acinetobacter nosocomialis, Acinetobacter pitti and Acinetobacter seifertii.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.
In enzymology, an alcohol dehydrogenase (acceptor) (EC 1.1.99.8) is an enzyme that catalyzes the chemical reaction

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Oenin is an anthocyanin. It is the 3-glucoside of malvidin. It is one of the red pigments found in the skin of purple grapes and in wine.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.
Bradyrhizobium japonicum is a species of legume-root nodulating, microsymbiotic nitrogen-fixing bacteria. The species is one of many Gram-negative, rod-shaped bacteria commonly referred to as rhizobia. Within that broad classification, which has three groups, taxonomy studies using DNA sequencing indicate that B. japonicum belongs within homology group II.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
B type proanthocyanidins are a specific type of proanthocyanidin, which are a class of flavanoids. They are oligomers of flavan-3-ols.
Acinetobacter nosocomialis is a gram-negative, strictly aerobic bacterium from the genus Acinetobacter isolated from a patient at MetroHealth in Cleveland, Ohio. Acinetobacter nosocomialis belongs to the Acinetobacter calcoaceticus-baumannii complex.
Acinetobacter pittii is a Gram-negative, oxidase-negative, catalase-positive, strictly aerobic, nonmotile, diplococcoid rod bacterium from the genus Acinetobacter. DNA-DNA hybridization studies have been used to identify DNA groups within the genus Acinetobacter and A. pittii belongs to the Acinetobacter calcoaceticus-baumannii complex. The specific epithet pittii is named after the British microbiologist Tyrone Pitt.

Acinetobacter baylyi is a bacterial species of the genus Acinetobacter. The species naming designation was given after the discovery of strains in activated sludge in Victoria, Australia, in 2003. A. baylyi is named after the late Dr. Ronald Bayly, an Australian microbiologist who contributed significantly to research on aromatic compound catabolism in diverse bacteria, including strains of Pseudomonas, Alcaligenes, and Acinetobacter. The new species designation in 2003 was found to apply to an already well-studied Acinetobacter strain known as ADP1, a derivative of a soil isolate characterized in 1969. Strain ADP1 was previously designated Acinetobacter sp. and Acinetobacter calcoaceticus. Research, particularly in the field of genetics, has established A. baylyi as a model organism.












