Punicalagin (Pyuni-cala-jen) is an ellagitannin, a type of phenolic compound. It is found as alpha and beta isomers in pomegranates, Terminalia catappa, Terminalia myriocarpa, and in Combretum molle, the velvet bushwillow, a plant species found in South Africa. These three genera are all Myrtales and the last two are both Combretaceae.

Terminalia chebula, commonly known as black- or chebulic myrobalan, is a species of Terminalia, native to South Asia from Pakistan, India and Nepal east to southwest China (Yunnan), and south to Sri Lanka, Malaysia, and Vietnam.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Ethyl gallate is a food additive with E number E313. It is the ethyl ester of gallic acid. Ethyl gallate is added to food as an antioxidant.

Hyperoside is a chemical compound. It is the 3-O-galactoside of quercetin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

Paeonia anomala is a species of herbaceous perennial flowering plant in the family Paeoniaceae. This peony is ½-1 m high, with a thick irregular taproot and thin side roots. The deeply incised leaves have leaflets which are themselves divided in fine segments. It flowers in early summer, almost always with only one fully developed flower per stem, magenta-red or rarely pink or white. The species occurs in a zone between northern European Russia and northern Mongolia and south to the Tien Shan Mountains.

Quercus infectoria or the Aleppo oak is a species of oak well known for producing galls that have been traditionally used for centuries in Asia medicinally while also used in softening leather and in making black dye and ink.
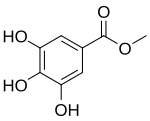
Bergenin, alias cuscutin, is trihydroxybenzoic acid glycoside. It is the C-glycoside of 4-O-methyl gallic acid. It possesses an O-demethylated derivative called norbergenin. These are chemical compounds and drugs of Ayurveda, commonly known as Paashaanbhed. It shows a potent immunomodulatory effect.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
Tergallic acids are trimers of gallic acid, often found naturally in the form of glycosides. Tergallic acid O- or C-glucosides that can be found in acorns of several Quercus (oak) species. The dehydrated tergallic acid C-glucoside and tergallic acid O-glucoside can be characterised in the acorns of Quercus macrocarpa. Dehydrated tergallic-C-glucoside can be found in the cork from Quercus suber.

Flavogallonic acid is a hydrolysable tannin that can be found in valonea oak in chestnut wood or in Terminalia myriocarpa.

Miquelianin is a flavonol glucuronide, a type of phenolic compound present in wine, in species of St John's wort, like Hypericum hirsutum, in Nelumbo nucifera or in green beans.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.

Terminalia myriocarpa, the East Indian almond, is a tree species in the genus Terminalia found in Southeast Asia.

Sanguiin H-6 is an ellagitannin.

N-Methylserotonin is a tryptamine alkaloid. Chemically, it is a derivative of serotonin in which a methyl group resides at its alkyl amine. It is also called Nω-methylserotonin (Nω-methyl-5-hydroxytryptamine) to distinguish it from tryptamine-derived compounds in which a methyl group is bonded to the nitrogen atom of the indole group.
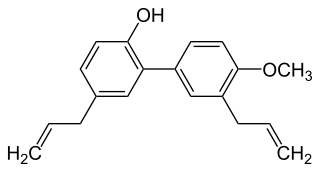
4-O-Methylhonokiol is a neolignan, a type of phenolic compound. It is found in the bark of Magnolia grandiflora and in M. virginiana flowers.
Geranium niveum is a plant species in the genus Geranium.