
Prunus is a genus of trees and shrubs, which includes the fruits plums, cherries, peaches, nectarines, apricots, and almonds.

Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

Terminalia chebula, commonly known as black- or chebulic myrobalan, is a species of Terminalia, native to South Asia from Pakistan, India and Nepal east to southwest China (Yunnan), and south to Sri Lanka, Malaysia, and Vietnam.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.
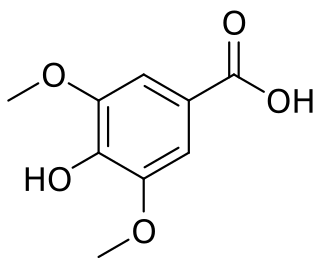
Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel of tea. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Vitisin B is a natural phenol found in red wines. It is a pyranoanthocyanin.

Wine is a complex mixture of chemical compounds in a hydro-alcoholic solution with a pH around 4. The chemistry of wine and its resultant quality depend on achieving a balance between three aspects of the berries used to make the wine: their sugar content, acidity and the presence of secondary compounds. Vines store sugar in grapes through photosynthesis, and acids break down as grapes ripen. Secondary compounds are also stored in the course of the season. Anthocyanins give grapes a red color and protection against ultraviolet light. Tannins add bitterness and astringency which acts to defend vines against pests and grazing animals.
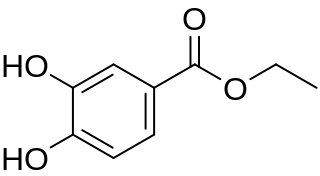
Ethyl protocatechuate is a phenolic compound. It can be found in the peanut seed testa. It is also present in wine. It is the ethylic ester of protocatechuic acid.

Delphinidin 3-O-(6-p-coumaroyl)glucoside is a p-coumaroylated anthocyanin. It can be found in some red Vitis vinifera grape cultivars and in red wine.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.

Terminalia myriocarpa, the East Indian almond, is a tree species in the genus Terminalia found in Southeast Asia.

Sanguiin H-6 is an ellagitannin.

Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.

Urolithin B (UB) is an urolithin, a type of phenolic compounds produced in the human gut after absorption of ellagitannins-containing food such as pomegranate, strawberries, red raspberries, walnuts or oak-aged red wine. Urolithin B is found in the urine in the form of urolithin B glucuronide.




















