Saponins, also selectively referred to as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort, a flowering plant, the soapbark tree and soybeans. They are used in soaps, medicines, fire extinguishers, speciously as dietary supplements, for synthesis of steroids, and in carbonated beverages. Structurally, they are glycosides, sugars bonded to another organic molecule, usually a steroid or triterpene, a steroid building block. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Bergenia is a genus of ten species of flowering plants in the family Saxifragaceae, native to central Asia, from Afghanistan to China and the Himalayan region.

Sida cordifolia is a perennial subshrub of the mallow family Malvaceae native to India. It has naturalized throughout the world, and is considered an invasive weed in Africa, Australia, the southern United States, Hawaiian Islands, New Guinea, and French Polynesia. The specific name, cordifolia, refers to the heart-shaped leaf.

Terminalia chebula, commonly known as black- or chebulic myrobalan, is a species of Terminalia, native to South Asia from India and Nepal east to southwest China (Yunnan), and south to Sri Lanka, Malaysia, and Vietnam.

Dryobalanops aromatica, commonly known as Borneo camphor, camphor tree, Malay camphor, or Sumatran camphor, is a species of critically endangered plant in the family Dipterocarpaceae. The species name aromatica is derived from Latin and refers to the smell of the dammar (resin). This species was one of the main sources of camphor and attracted early Arab traders to Borneo, at that time being worth more than gold, and used for incense and perfumes.

Afzelechin is a flavan-3-ol, a type of flavonoid. It can be found in Bergenia ligulata. It exists as at least 2 major epimers.

Bergenia ligulata is a plant belonging to the family Saxifragaceae and the genus Bergenia. It is plant is sometimes treated as a form of Bergenia ciliata. It is mostly found in temperate regions of the Himalayas from Kashmir to Bhutan and in Khasia hills at 1,500 m (4,900 ft) altitude.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

α-Viniferin is a stilbene trimer. It can be isolated from Caragana chamlagu and from Caragana sinica and from the stem bark of Dryobalanops aromatica. It is also present in relation to resistance to Botrytis cinerea and Plasmopara viticola in Vitis vinifera and Vitis riparia.It has been shown to inhibit acetylcholinesterase.
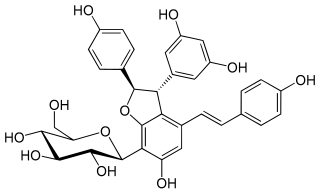
Diptoindonesin A is a C-glucoside of ε-viniferin isolated from the two Dipterocarpaceae Shorea seminis and Dryobalanops aromatica.

Artemisia herba-alba, the white wormwood, is a perennial shrub in the genus Artemisia that grows commonly on the dry steppes of the Mediterranean regions in Northern Africa, Western Asia and Southwestern Europe. It is used as an antiseptic and antispasmodic in herbal medicine.

Verbascoside is a caffeoyl phenylethanoid glycoside in which the phenylpropanoid caffeic acid and the phenylethanoid hydroxytyrosol form an ester and an ether bond respectively, to the rhamnose part of a disaccharide, namely β-(3′,4′-dihydroxyphenyl)ethyl-O-α-L-rhamnopyranosyl(1→3)-β-D-(4-O-caffeoyl)-glucopyranoside.

Bergenia ciliata is a plant species in the genus Bergenia, deciduous in USDA Zones 5 to 7, but usually remain semi-evergreen south of Zone 7. It is found in Northern India in Uttarakhand and Himachal Pradesh. This flower is related to the famous Phool Dei Festival celebrated in Uttarakhand. It is commonly known in India as Pathar phor buti. Also found in mountain areas of West Bengal, like Kalimpong, and Darjeeling. Afghanistan, south Tibet, Northern Nepal, Bhutan.

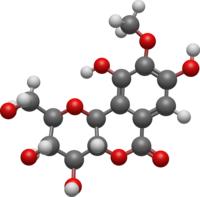
Norbergenin is a chemical compound. It is the O-demethylated derivative of bergenin. It can be isolated from rhizomes of Bergenia stracheyi.

Bergenia stracheyi is a plant species in the genus Bergenia found in the Western Himalayas, from 2700 to 4700 m, Afghanistan and Tajikistan.

Mallotus japonicus, also known as East Asian mallotus, the food wrapper plant or "Akamegashiwa" in Japanese, is a plant species in the genus Mallotus native to China. It is also found in Japan and Korea. This species was first described in 1865, its name was verified by AAS Systematic Botanists on October 2, 2015.

Mallotusinic acid is a hydrolysable tannin found in the bark of Mallotus japonicus. It is more generally present in Geraniales.

Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.

Mallojaponin is a hydrolysable tannin found in the bark of Mallotus japonicus. This compound contains the moiety elaeocarpusinic acid, an oxidized hexahydroxydiphenic acid group which reacted with a dehydroascorbic acid molecule. It also contains a valoneic acid and a gallic acid moieties linked to a glucose molecule.

Styrax japonicus, also known as the Japanese snowbell, is a species of flowering plant in the family Styracaceae, native to Korea, Japan, and Southern China. Growing to 12 m (39 ft) tall by 8 m (26 ft) broad, it is a graceful, spreading deciduous tree with oval, upward-facing leaves which occasionally turn yellow or orange before falling in autumn. Masses of slightly fragrant, bell-shaped white flowers hang from the branches in summer, followed by fruits (drupes) which resemble olives in both shape and colour.