Related Research Articles

Glycolysis is the metabolic pathway that converts glucose into pyruvate, and in most organisms, occurs in the liquid part of cells, the cytosol. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.
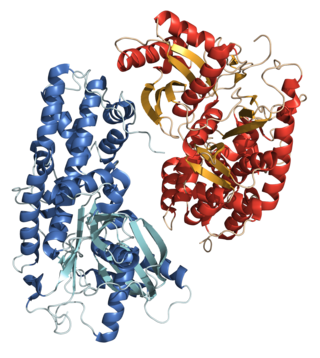
A hexokinase is an enzyme that phosphorylates hexoses, forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate.

Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.
Phosphotransferases are a category of enzymes that catalyze phosphorylation reactions. The general form of the reactions they catalyze is:
A tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone functional group in position 2 (ketotetroses).

Myophosphorylase or glycogen phosphorylase, muscle associated (PYGM) is the muscle isoform of the enzyme glycogen phosphorylase and is encoded by the PYGM gene. This enzyme helps break down glycogen into glucose-1-phosphate, so it can be used within the muscle cell. Mutations in this gene are associated with McArdle disease, a glycogen storage disease of muscle.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.
In enzymology, a β-phosphoglucomutase is an enzyme that catalyzes the chemical reaction

In enzymology, a phosphoacetylglucosamine mutase is an enzyme that catalyzes the chemical reaction

In enzymology, a phosphoenolpyruvate mutase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoglucosamine mutase is an enzyme that catalyzes the chemical reaction
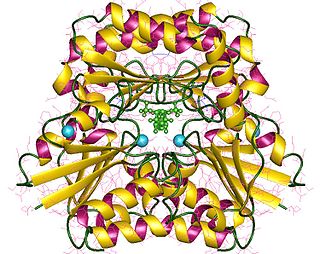
In enzymology, a phosphomannomutase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphopentomutase is an enzyme that catalyzes the chemical reaction
In enzymology, a glucose-1-phosphate phosphodismutase is an enzyme that catalyzes the chemical reaction
In enzymology, a glycerol-3-phosphate-glucose phosphotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoglucokinase is an enzyme that catalyzes the chemical reaction
In enzymology, a polyphosphate-glucose phosphotransferase is an enzyme that catalyzes the chemical reaction.
In enzymology, a riboflavin phosphotransferase is an enzyme that catalyzes the chemical reaction
N,N'-diacetylchitobiose phosphorylase is an enzyme with the systematic name N,N'-diacetylchitobiose:phosphate N-acetyl-D-glucosaminyltransferase. This enzyme was found in the genus Vibrio initially but has now been found to be taken up by Escherichia coli as well as many other bacteria. One study shows that Escherichia coli can replicate on a medium that is just composed of GlcNAc a product of phosphorylation of N,N'-diacetylchitobiose as the sole source of carbon. Because E. coli can go on this medium, the enzyme is present. The enzyme has also been found in multiple eukaryotic cells as well, especially in eukaryotes that make chitin and break chitin down. It is believed that N,N'-diacetylchitobiose phosphorylase is an integral part of the phosphoenolpyruvate:glucose phosphotransferase system (PTS). It is assumed that it is involved with Enzyme Complex II of the PTS and is involved with the synthesis of chitin. The enzyme is specific for N,N'-diacetylchitobiose.
Undecaprenyl-phosphate glucose phosphotransferase is an enzyme with systematic name UDP-glucose:ditrans,octacis-undecaprenyl-phosphate glucose phosphotransferase. This enzyme catalyses the following chemical reaction
References
- FUJIMOTO A, INGRAM P, SMITH RA (1965). "D-GLUCOSE-I-PHOSPHATE:D-GLUCOSE-6-PHOSPHOTRANSFERASE". Biochim. Biophys. Acta. 96: 91–101. doi:10.1016/0005-2787(65)90613-1. PMID 14285271.
- Boyer, P.D. (Ed.), The Enzymes, 3rd ed., vol. 6, 1972, p. 407-477.