The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge.

Praseodymium is a chemical element; it has symbol Pr and the atomic number 59. It is the third member of the lanthanide series and is considered one of the rare-earth metals. It is a soft, silvery, malleable and ductile metal, valued for its magnetic, electrical, chemical, and optical properties. It is too reactive to be found in native form, and pure praseodymium metal slowly develops a green oxide coating when exposed to air.
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.
In chemistry, the valence or valency of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given element typically forms. For a specified compound the valence of an atom is the number of bonds formed by the atom. Double bonds are considered to be two bonds, and triple bonds to be three. In most compounds, the valence of hydrogen is 1, of oxygen is 2, of nitrogen is 3, and of carbon is 4. Valence is not to be confused with the related concepts of the coordination number, the oxidation state, or the number of valence electrons for a given atom.

Magnesium nitride, which possesses the chemical formula Mg3N2, is an inorganic compound of magnesium and nitrogen. At room temperature and pressure it is a greenish yellow powder.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

A sextuple bond is a type of covalent bond involving 12 bonding electrons and in which the bond order is 6. The only known molecules with true sextuple bonds are the diatomic dimolybdenum (Mo2) and ditungsten (W2), which exist in the gaseous phase and have boiling points of 4,639 °C (8,382 °F) and 5,930 °C (10,710 °F) respectively.
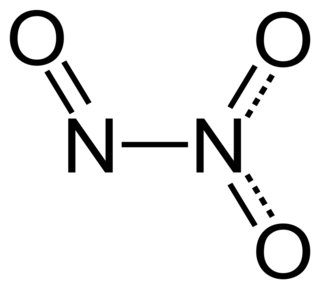
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):
The nitridoborates are chemical compounds of boron and nitrogen with metals. These compounds are typically produced at high temperature by reacting hexagonal boron nitride with metal nitrides or by metathesis reactions involving nitridoborates. A wide range of these compounds have been made involving lithium, alkaline earth metals and lanthanides, and their structures determined using crystallographic techniques such as X-ray crystallography. Structurally one of their interesting features is the presence of polyatomic anions of boron and nitrogen where the geometry and the B–N bond length have been interpreted in terms of π-bonding.
Pauling's principle of electroneutrality states that each atom in a stable substance has a charge close to zero. It was formulated by Linus Pauling in 1948 and later revised. The principle has been used to predict which of a set of molecular resonance structures would be the most significant, to explain the stability of inorganic complexes and to explain the existence of π-bonding in compounds and polyatomic anions containing silicon, phosphorus or sulfur bonded to oxygen; it is still invoked in the context of coordination complexes. However, modern computational techniques indicate many stable compounds have a greater charge distribution than the principle predicts.

In coordination chemistry and organometallic chemistry, transition metal imido complexes is a coordination compound containing an imido ligand. Imido ligands can be terminal or bridging ligands. The parent imido ligand has the formula NH, but most imido ligands have alkyl or aryl groups in place of H. The imido ligand is generally viewed as a dianion, akin to oxide.

Yttrium(III) nitrate is an inorganic compound, a salt with the formula Y(NO3)3. The hexahydrate is the most common form commercially available.
The boroselenites are heteropoly anion chemical compounds containing selenite and borate groups linked by common oxygen atoms. They are not to be confused with the boroselenates with have a higher oxidation state for selenium, and extra oxygen. If selenium is replaced by sulfur, it would be a borosulfite. Boroselenites are distinct from selenoborates in which selenium replaces oxygen in borate, or perselenoborates which contain Se-Se bonds as well as Se-B bonds. The metal boroselenites were only discovered in 2012.
Sulfidogermanates or thiogermanates are chemical compounds containing anions with sulfur atoms bound to germanium. They are in the class of chalcogenidotetrelates. Related compounds include thiosilicates, thiostannates, selenidogermanates, telluridogermanates and selenidostannates.
Neodymium nickelate is a nickelate of neodymium with a chemical formula NdNiO3. In this compound, the neodymium atom is in the +3 oxidation state.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.
Neodymium(III) nitride is a chemical compound of neodymium and nitrogen with the formula NdN in which neodymium exhibits the +3 oxidation state and nitrogen exhibits the -3 oxidation state. It is ferromagnetic, like gadolinium(III) nitride, terbium(III) nitride and dysprosium(III) nitride. Neodymium(III) nitride is not usually stoichiometric, and it is very hard to create pure stoichiometric neodymium nitride.







