Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
The aldol reaction is a reaction in organic chemistry that combines two carbonyl compounds to form a new β-hydroxy carbonyl compound. Its simplest form might involve the nucleophilic addition of an enolized ketone to another:

An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
The chiral pool is a "collection of abundant enantiopure building blocks provided by nature" used in synthesis. In other words, a chiral pool would be a large quantity of common organic enantiomers. Contributors to the chiral pool are amino acids, sugars, and terpenes. Their use improves the efficiency of total synthesis. Not only does the chiral pool contribute a premade carbon skeleton, their chirality is usually preserved in the remainder of the reaction sequence.

The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which a prochiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction.

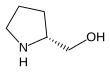
The CBS catalyst or Corey–Bakshi–Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and (3+2) cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available. It transfers its stereocenter to the catalyst which in turn is able to drive an organic reaction selectively to one of two possible enantiomers. This selectivity is due to steric strain in the transition state that develops for one enantiomer but not for the other.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special substituents. With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile. The negative charge in these structures is stored as an alkoxide anion. Such a nucleophilic addition is called a nucleophilic conjugate addition or 1,4-nucleophilic addition. The most important active alkenes are the aforementioned conjugated carbonyls and acrylonitriles.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with cyanide in the presence of ammonia. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used for the commercial production of racemic methionine from methional.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
The Hajos–Parrish–Eder–Sauer–Wiechert and Barbas-List reactions in organic chemistry are a family of proline-catalysed asymmetric aldol reactions.

Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols. The reagent is often depicted as a monomer but like most hydroboranes, it is dimeric with B-H-B bridges.

DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.

Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula BH3·S(CH3)2. It is an adduct between borane molecule and dimethyl sulfide molecule. It is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction of THF to tributyl borate. BMS is soluble in most aprotic solvents.
The Schöllkopf method or Schöllkopf Bis-Lactim Amino Acid Synthesis is a method in organic chemistry for the asymmetric synthesis of chiral amino acids. The method was established in 1981 by Ulrich Schöllkopf. In it glycine is a substrate, valine a chiral auxiliary and the reaction taking place an alkylation.
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen.

The Enders SAMP/RAMP hydrazone alkylation reaction is an asymmetric carbon-carbon bond formation reaction facilitated by pyrrolidine chiral auxiliaries. It was pioneered by E. J. Corey and Dieter Enders in 1976, and was further developed by Enders and his group. This method is usually a three-step sequence. The first step is to form the hydrazone between (S)-1-amino-2-methoxymethylpyrrolidine (SAMP) or (R)-1-amino-2-methoxymethylpyrrolidine (RAMP) and a ketone or aldehyde. Afterwards, the hydrazone is deprotonated by lithium diisopropylamide (LDA) to form an azaenolate, which reacts with alkyl halides or other suitable electrophiles to give alkylated hydrazone species with the simultaneous generation of a new chiral center. Finally, the alkylated ketone or aldehyde can be regenerated by ozonolysis or hydrolysis.
Proline organocatalysis is the use of proline as an organocatalyst in organic chemistry. This theme is often considered the starting point for the area of organocatalysis, even though early discoveries went unappreciated. Modifications, such as MacMillan’s catalyst and Jorgensen's catalysts, proceed with excellent stereocontrol.