
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.

The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula XY(Si,Al)2O6, where X represents calcium (Ca), sodium (Na), iron or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron. Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes.

Armalcolite is a titanium-rich mineral with the chemical formula (Mg,Fe2+)Ti2O5. It was first found at Tranquility Base on the Moon in 1969 during the Apollo 11 mission, and is named for Armstrong, Aldrin and Collins, the three Apollo 11 astronauts. Together with tranquillityite and pyroxferroite, it is one of three new minerals that were discovered on the Moon. Armalcolite was later identified at various locations on Earth and has been synthesized in the laboratory. (Tranquillityite and pyroxferroite were also later found at various locations on Earth). The synthesis requires low pressures, high temperatures and rapid quenching from about 1,000 °C to the ambient temperature. Armalcolite breaks down to a mixture of magnesium-rich ilmenite and rutile at temperatures below 1,000 °C, but the conversion slows down with cooling. Because of this quenching requirement, armalcolite is relatively rare and is usually found in association with ilmenite and rutile, among other minerals.

Cristobalite is a mineral polymorph of silica that is formed at very high temperatures. It has the same chemical formula as quartz, SiO2, but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members of the quartz group, which also include coesite, tridymite and stishovite. It is named after Cerro San Cristóbal in Pachuca Municipality, Hidalgo, Mexico.

Coesite is a form (polymorph) of silicon dioxide (SiO2) that is formed when very high pressure (2–3 gigapascals), and moderately high temperature (700 °C, 1,300 °F), are applied to quartz. Coesite was first synthesized by Loring Coes, Jr., a chemist at the Norton Company, in 1953.

Peridotite ( PERR-ih-doh-tyte, pə-RID-ə-) is a dense, coarse-grained igneous rock consisting mostly of the silicate minerals olivine and pyroxene. Peridotite is ultramafic, as the rock contains less than 45% silica. It is high in magnesium (Mg2+), reflecting the high proportions of magnesium-rich olivine, with appreciable iron. Peridotite is derived from Earth's mantle, either as solid blocks and fragments, or as crystals accumulated from magmas that formed in the mantle. The compositions of peridotites from these layered igneous complexes vary widely, reflecting the relative proportions of pyroxenes, chromite, plagioclase, and amphibole.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Jadeite is a pyroxene mineral with composition NaAlSi2O6. It is hard (Mohs hardness of about 6.5 to 7.0), very tough, and dense, with a specific gravity of about 3.4. It is found in a wide range of colors, but is most often found in shades of green or white. Jadeite is formed only in the subduction zones of continental margins, where rock undergoes metamorphism at high pressure but relatively low temperature.

The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite.
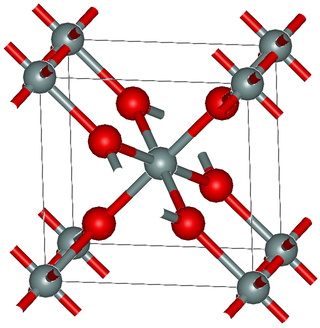
Stishovite is an extremely hard, dense tetragonal form (polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in the lower mantle.

Fractional crystallization, or crystal fractionation, is one of the most important geochemical and physical processes operating within crust and mantle of a rocky planetary body, such as the Earth. It is important in the formation of igneous rocks because it is one of the main processes of magmatic differentiation. Fractional crystallization is also important in the formation of sedimentary evaporite rocks or simply fractional crystallization is the removal of early formed crystals from an Original homogeneous magma so that the crystals are prevented from further reaction with the residual melt.
Zussmanite is a hydrated iron-rich silicate mineral with the chemical formula K(Fe2+,Mg,Mn)13[AlSi17O42](OH)14. It occurs as pale green crystals with perfect cleavage.

Magmatic water, also known as juvenile water, is an aqueous phase in equilibrium with minerals that have been dissolved by magma deep within the Earth's crust and is released to the atmosphere during a volcanic eruption. It plays a key role in assessing the crystallization of igneous rocks, particularly silicates, as well as the rheology and evolution of magma chambers. Magma is composed of minerals, crystals and volatiles in varying relative natural abundance. Magmatic differentiation varies significantly based on various factors, most notably the presence of water. An abundance of volatiles within magma chambers decreases viscosity and leads to the formation of minerals bearing halogens, including chloride and hydroxide groups. In addition, the relative abundance of volatiles varies within basaltic, andesitic, and rhyolitic magma chambers, leading to some volcanoes being exceedingly more explosive than others. Magmatic water is practically insoluble in silicate melts but has demonstrated the highest solubility within rhyolitic melts. An abundance of magmatic water has been shown to lead to high-grade deformation, altering the amount of δ18O and δ2H within host rocks.

Clinopyroxene thermobarometry is a scientific method that uses the mineral clinopyroxene to determine the temperature and pressure of the magma when the mineral crystalized. Clinopyroxene is found in many igneous rocks, so the method can be used to determine information about the entire rock. Many different minerals can be used for geothermobarometry, but clinopyroxene is especially useful because it's a common phenocryst in igneous rocks and easy to identify, and the crystallization of jadeite, a type of clinopyroxene, implies a growth in molar volume, making it a good indicator of pressure.

Pyroxmangite has the general chemical formula of MnSiO3. It is the high-pressure, low-temperature dimorph of rhodonite.

Kanoite is a light pinkish brown silicate mineral that is found in metamorphic rocks. It is an inosilicate and has a chemical formula of (Mg,Mn2+)2Si2O6. It is a member of pyroxene group and clinopyroxene subgroup.
Tranquillityite is silicate mineral with formula (Fe2+)8Ti3Zr2 Si3O24. It is mostly composed of iron, oxygen, silicon, zirconium and titanium with smaller fractions of yttrium and calcium. It is named after the Mare Tranquillitatis (Sea of Tranquility), the place on the Moon where the rock samples were found during the 1969 Apollo 11 mission. It was the last mineral brought from the Moon which was thought to be unique, with no counterpart on Earth, until it was discovered in Australia in 2011.

Dorrite is a silicate mineral that is isostructural to the aenigmatite group. It is most chemically similar to the mineral rhönite [Ca2Mg5Ti(Al2Si4)O20], made distinct by a lack of titanium (Ti) and the presence of Fe3+. Dorrite is named for Dr. John (Jack) A. Dorr, a late professor at the University of Michigan that researched in outcrops where dorrite was found in 1982. This mineral is sub-metallic resembling colors of brownish-black, dark brown, to reddish brown.

Yoshiokaite, a mineral formed as shocked crystal fragments in devitrified glass, was discovered in lunar regolith breccia collected from a trench by the Apollo 14 crew in 1971. Although there have been other minerals that have been originally discovered on the Moon, yoshiokaite is the first new mineral with origin related to lunar highlands. Yoshiokaite is considered to be a member of the feldspathoid group.



















