Related Research Articles
Zygomycosis is the broadest term to refer to infections caused by bread mold fungi of the zygomycota phylum. However, because zygomycota has been identified as polyphyletic, and is not included in modern fungal classification systems, the diseases that zygomycosis can refer to are better called by their specific names: mucormycosis, phycomycosis and basidiobolomycosis. These rare yet serious and potentially life-threatening fungal infections usually affect the face or oropharyngeal cavity. Zygomycosis type infections are most often caused by common fungi found in soil and decaying vegetation. While most individuals are exposed to the fungi on a regular basis, those with immune disorders (immunocompromised) are more prone to fungal infection. These types of infections are also common after natural disasters, such as tornadoes or earthquakes, where people have open wounds that have become filled with soil or vegetative matter.
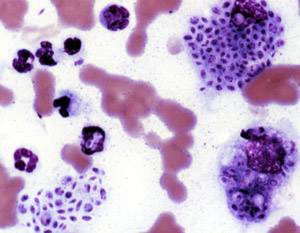
Sporotrichosis, also known as rose handler's disease, is a fungal infection that may be localised to skin, lungs, bone and joint, or become systemic. It presents with firm painless nodules that later ulcerate. Following initial exposure to Sporothrix schenckii, the disease typically progresses over a period of a week to several months. Serious complications may develop in people who have a weakened immune system.
Phycomycosis is an uncommon condition of the gastrointestinal tract and skin most commonly found in dogs and horses. The condition is caused by a variety of molds and fungi, and individual forms include pythiosis, zygomycosis, and lagenidiosis. Pythiosis is the most common type and is caused by Pythium, a type of water mould. Zygomycosis can also be caused by two types of zygomycetes, Entomophthorales and Mucorales. The latter type of zygomycosis is also referred to as mucormycosis. Lagenidiosis is caused by a Lagenidium species, which like Pythium is a water mould. Since both pythiosis and lagenidiosis are caused by organisms from the class Oomycetes, they are sometimes collectively referred to as oomycosis.

Basidiobolus ranarum is a filamentous fungus with worldwide distribution. The fungus was first isolated by Eidam in 1886. It can saprophytically live in the intestines of mainly cold-blooded vertebrates and on decaying fruits and soil. The fungus prefers glucose as a carbon source and grows rapidly at room temperature. Basidiobolus ranarum is also known as a cause of subcutaneous zygomycosis, usually causing granulomatous infections on a host's limbs. Infections are generally geographically limited to tropical and subtropical regions such as East and West Africa. Subcutaneous zygomycosis caused by B. ranarum is a rare disease and predominantly affects children and males. Common subcutaneous zygomycosis shows characteristic features and is relatively easy to be diagnosed; while, certain rare cases might show non-specific clinical features that might pose a difficulty on its identification. Although disease caused by this fungus is known to resolve spontaneously on its own, there are a number of treatments available.

Apophysomyces is a genus of filamentous fungi that are commonly found in soil and decaying vegetation. Species normally grow in tropical to subtropical regions.

Mucor mucedo, commonly known as the common pinmould, is a fungal plant pathogen and member of the phylum Mucoromycota and the genus Mucor. Commonly found on soil, dung, water, plants and moist foods, Mucor mucedo is a saprotrophic fungus found world-wide with 85 known strains. It is often mistaken for Rhizopus rots on fruits due to similar mould growth shape and colour. Contrastingly, however, Mucor mucedo is found to grow on a wide range of stored grains and plants, including cucumber and tomato. Discovered in Italy in 1729 by P.A. Micheli and later noted by Carl Linnaeus in 1753 in the Species Plantarum, Mucor mucedo was originally classified as Mucor vulgaris by Micheli but later classified synonymous under name Mucor mucedo. The species was redescribed as Ascophora mucedo by H.J. Tode in 1790 but this type resided in a stoloniferous habitat and was later made the type of new genus Rhizopus.

Setosphaeria rostrata is a heat tolerant fungus with an asexual reproductive form (anamorph) known as Exserohilum rostratum. This fungus is a common plant pathogen, causing leaf spots as well as crown rot and root rot in grasses. It is also found in soils and on textiles in subtropical and tropical regions. Exserohilum rostratum is one of the 35 Exserohilum species implicated uncommonly as opportunistic pathogens of humans where it is an etiologic agent of sinusitis, keratitis, skin lesions and an often fatal meningoencephalitis. Infections caused by this species are most often seen in regions with hot climates like Israel, India and the southern USA.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.

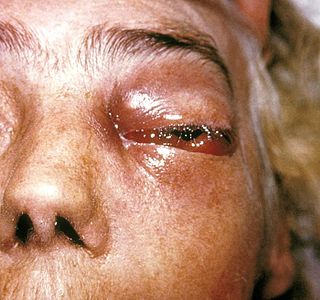
Mucormycosis, also known as black fungus, is a serious fungal infection that comes under fulminant fungal sinusitis, usually in people who are immunocompromised. It is curable only when diagnosed early. Symptoms depend on where in the body the infection occurs. It most commonly infects the nose, sinuses, eyes and brain resulting in a runny nose, one-sided facial swelling and pain, headache, fever, blurred vision, bulging or displacement of the eye (proptosis), and tissue death. Other forms of disease may infect the lungs, stomach and intestines, and skin.
Entomophthoramycosis is a mycosis caused by Entomophthorales.
Rhizomucor pusillus is a species of Rhizomucor. It can cause disease in humans. R. pusillus is a grey mycelium fungi most commonly found in compost piles. Yellow-brown spores grow on a stalk to reproduce more fungal cells.

Exophiala dermatitidis is a thermophilic black yeast, and a member of the Herpotrichiellaceae. While the species is only found at low abundance in nature, metabolically active strains are commonly isolated in saunas, steam baths, and dish washers. Exophiala dermatitidis only rarely causes infection in humans, however cases have been reported around the world. In East Asia, the species has caused lethal brain infections in young and otherwise healthy individuals. The fungus has been known to cause cutaneous and subcutaneous phaeohyphomycosis, and as a lung colonist in people with cystic fibrosis in Europe. In 2002, an outbreak of systemic E. dermatitidis infection occurred in women who had received contaminated steroid injections at North Carolina hospitals.

Apophysomyces variabilis is an emerging fungal pathogen that can cause serious and sometimes fatal infection in humans. This fungus is a soil-dwelling saprobe with tropical to subtropical distribution. It is a zygomycete that causes mucormycosis, an infection in humans brought about by fungi in the order Mucorales. Infectious cases have been reported globally in locations including the Americas, Southeast Asia, India, and Australia. Apophysomyces variabilis infections are not transmissible from person to person.

Lichtheimia corymbifera is a thermophilic fungus in the phylum Zygomycota. It normally lives as a saprotrophic mold, but can also be an opportunistic pathogen known to cause pulmonary, CNS, rhinocerebral, or cutaneous infections in animals and humans with impaired immunity.
Scedosporiosis is the general name for any mycosis - i.e., fungal infection - caused by a fungus from the genus Scedosporium. Current population-based studies suggest Scedosporium prolificans and Scedosporium apiospermum to be among the most common infecting agents from the genus, although infections caused by other members thereof are not unheard of. The latter is an asexual form (anamorph) of another fungus, Pseudallescheria boydii. The former is a “black yeast”, currently not characterized as well, although both of them have been described as saprophytes.
Histoplasma duboisii is a saprotrophic fungus responsible for the invasive infection known as African histoplasmosis. This species is a close relative of Histoplasma capsulatum, the agent of classical histoplasmosis, and the two occur in similar habitats. Histoplasma duboisii is restricted to continental Africa and Madagascar, although scattered reports have arisen from other places usually in individuals with an African travel history. Like, H. capsulatum, H. duboisii is dimorphic – growing as a filamentous fungus at ambient temperature and a yeast at body temperature. It differs morphologically from H. capsulatum by the typical production of a large-celled yeast form. Both agents cause similar forms of disease, although H. duboisii predominantly causes cutaneous and subcutaneous disease in humans and non-human primates. The agent responds to many antifungal drug therapies used to treat serious fungal diseases.
Cunninghamella bertholletiae is a species of zygomycetous fungi in the order Mucorales. It is found globally, with increased prevalence in Mediterranean and subtropical climates. It typically grows as a saprotroph and is found in a wide variety of substrates, including soil, fruits, vegetables, nuts, crops, and human and animal waste. Although infections are still rare, C. betholletiae is emerging as an opportunistic human pathogen, predominantly in immunocompromised people, leukemia patients, and people with uncontrolled diabetes. Cunninghamella bertholletiae infections are often highly invasive, and can be more difficult to treat with antifungal drugs than infections with other species of the Mucorales, making prompt and accurate recognition and diagnosis of mycoses caused by this fungus an important medical concern.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Chaetomium perlucidum is a neurotropic dematiaceous fungus that is naturally found in the soil, including in agricultural soil, and in the stems of dead plants. The fungus can also be found on the feathers of birds, manure, seeds, and even paper. It is able to thrive at temperatures of 35 and 42 °C.
Lichtheimia ramosa is a saprotrophic zygomycete, typically found in soil or dead plant material. It is a thermotolerant fungus that has also been known to act as an opportunistic pathogen–infecting both humans and animals.
References
- 1 2 3 4 Vega W; Orellana M; Zaror L; Gené J; Guarro J. (2006). "Saksenaea vasiformis infections: case report and literature review". Mycopathologia. 162 (4): 289–94. doi:10.1007/s11046-006-0061-6. PMID 17039275. S2CID 4672160.
- ↑ Padmaja IJ; Ramani TV; Kalyani S. (2006). "Cutaneous zygomycosis: necrotising fascitis due to Saksenaea vasiformis". Indian Journal of Medical Microbiology. 24 (1): 58–60. doi: 10.1016/S0255-0857(21)02474-9 . hdl: 1807/7251 . PMID 16505559.
- 1 2 3 4 5 6 7 8 9 10 11 12 Ribes, J. A.; Vanover-Sams, C. L.; Baker, D. J. (1 April 2000). "Zygomycetes in human disease". Clinical Microbiology Reviews. 13 (2): 236–301. doi:10.1128/CMR.13.2.236. PMC 100153 . PMID 10756000.
- 1 2 3 4 5 6 7 8 9 10 Kaushik, Robin; Chander, Jagdish; Gupta, Sanjay; Sharma, Rajeev; Punia, Rajpal Singh (1 April 2012). "Fatal Primary Cutaneous Zygomycosis Caused by Saksenaea vasiformis: Case report and review of literature". Surgical Infections. 13 (2): 125–129. doi:10.1089/sur.2010.078. PMID 22280152.
- ↑ Bashford, C; Yin, T; Pack, J (February 2002). "Necrotizing fasciitis: a model nursing care plan". Medsurg Nursing. 11 (1): 37–42, quiz 43. PMID 11901612.
- 1 2 3 4 5 Saksena, SB (1953). "A New Genus of the Mucorales". Mycologia. 45 (3): 426–436. doi:10.1080/00275514.1953.12024280. JSTOR 4547711.
- 1 2 3 Chakrabarti, A.; Kumar, P.; Padhye, A. A.; Chatha, L.; Singh, S. K.; Das, A.; Wig, J. D.; Kataria, R. N. (1 April 1997). "Primary Cutaneous Zygomycosis Due to Saksenaea vasiformis and Apophysomyces elegans". Clinical Infectious Diseases. 24 (4): 580–582. doi: 10.1093/clind/24.4.580 . PMID 9145731.
- 1 2 3 Alvarez, E.; Garcia-Hermoso, D.; Sutton, D. A.; Cano, J. F.; Stchigel, A. M.; Hoinard, D.; Fothergill, A. W.; Rinaldi, M. G.; Dromer, F.; Guarro, J. (6 October 2010). "Molecular Phylogeny and Proposal of Two New Species of the Emerging Pathogenic Fungus Saksenaea". Journal of Clinical Microbiology. 48 (12): 4410–4416. doi:10.1128/JCM.01646-10. PMC 3008438 . PMID 20926710.
- ↑ Robeck, T. R; Dalton, L. M (2002). "Saksenaea vasiformis and Apophysomyces elegans Zygomycotic Infections in Bottlenose Dolphins (Tursiops truncatus), a Killer Whale (Orcinus orca), and Pacific White-Sided Dolphins (Lagenorhynchus obliquidens)". Journal of Zoo and Wildlife Medicine. 33 (4): 356–366. doi:10.1638/1042-7260(2002)033[0356:SVAAEZ]2.0.CO;2. PMID 12564534. S2CID 29488757.
- ↑ Ellis, J. J; Ajello, L (1982). "An Unusual Source for Apophysomyces elegans and a Method for Stimulating Sporulation of Saksenaea vasiformis". Mycologia. 74 (1): 144–145. doi:10.2307/3792640. JSTOR 3792640.
- ↑ Adam, R. D.; Hunter, G.; DiTomasso, J.; Comerci, G. (1 July 1994). "Mucormycosis: Emerging Prominence of Cutaneous Infections". Clinical Infectious Diseases. 19 (1): 67–76. doi:10.1093/clinids/19.1.67. PMID 7948560.
- ↑ Ajello, Libero; Dean, David F; Irwin, Richard S (1976). "The Zygomycete Saksenaea vasiformis as a Pathogen of Humans with a Critical Review of the Etiology of Zygomycosis". Mycologia. 68 (1): 52–62. doi:10.2307/3758897. JSTOR 3758897. PMID 945456.
- ↑ Pritchard, RC; Muir, DB; Archer, KH; Beith, JM (1–15 Dec 1986). "Subcutaneous zygomycosis due to Saksenaea vasiformis in an infant". The Medical Journal of Australia. 145 (11–12): 630–1. doi:10.5694/j.1326-5377.1986.tb139516.x. PMID 3796372.
- 1 2 Torell, J; Cooper, BH; Helgeson, NG (July 1981). "Disseminated Saksenaea vasiformis infection". American Journal of Clinical Pathology. 76 (1): 116–21. doi:10.1093/ajcp/76.1.116. PMID 6942651.
- ↑ Lye, GR; Wood, G; Nimmo, G (November 1996). "Subcutaneous zygomycosis due to Saksenaea vasiformis: rapid isolate identification using a modified sporulation technique". Pathology. 28 (4): 364–5. doi:10.1080/00313029600169364. PMID 9007959. S2CID 22179099.
- ↑ Padhye, A.A.; Koshi, G.; Anandi, V.; Ponniah, J.; Sitaram, V.; Jacob, M.; Mathai, R.; Ajello, L.; Chandler, F.W. (February 1988). "First case of subcutaneous zygomycosis caused by Saksenaea vasiformis in India". Diagnostic Microbiology and Infectious Disease. 9 (2): 69–77. doi:10.1016/0732-8893(88)90099-5. PMID 3289825.
- ↑ Bearer, EA; Nelson, PR; Chowers, MY; Davis, CE (July 1994). "Cutaneous zygomycosis caused by Saksenaea vasiformis in a diabetic patient". Journal of Clinical Microbiology. 32 (7): 1823–4. doi:10.1128/jcm.32.7.1823-1824.1994. PMC 263809 . PMID 7929783.
- ↑ HILL, BD; BLACK, PF; KELLY, M; MUIR, D; DONALD, WAJ MC (1 July 1992). "Bovine cranial zygomycosis caused by Saksenaea vasiformis". Australian Veterinary Journal. 69 (7): 173–174. doi:10.1111/j.1751-0813.1992.tb07509.x. PMID 1445086.
- ↑ Salas, Valentina; Pastor, F. Javier; Calvo, Enrique; Sutton, Deanna; García-Hermoso, Dea; Mayayo, Emilio; Dromer, Françoise; Fothergill, Anette; Alvarez, Eduardo; Guarro, Josep (1 October 2012). "Experimental murine model of disseminated infection by Saksenaea vasiformis: successful treatment with posaconazole". Medical Mycology. 50 (7): 710–715. doi: 10.3109/13693786.2012.673137 . PMID 22458251.
- ↑ Prabhu, R. M.; Patel, R. (March 2004). "Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment". Clinical Microbiology and Infection. 10 (s1): 31–47. doi: 10.1111/j.1470-9465.2004.00843.x . PMID 14748801.
- ↑ Spellberg, B.; Edwards, J.; Ibrahim, A. (14 July 2005). "Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management". Clinical Microbiology Reviews. 18 (3): 556–569. doi:10.1128/CMR.18.3.556-569.2005. PMC 1195964 . PMID 16020690.