Related Research Articles
A transient ischemic attack (TIA), commonly known as a mini-stroke, is a minor stroke whose noticeable symptoms usually end in less than an hour. A TIA causes the same symptoms associated with a stroke, such as weakness or numbness on one side of the body, sudden dimming or loss of vision, difficulty speaking or understanding language, slurred speech, or confusion.
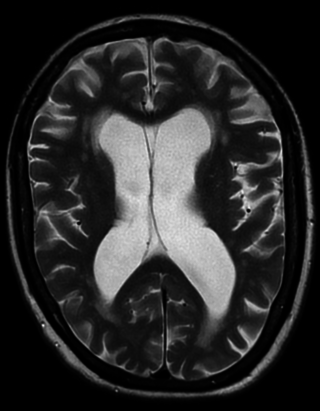
Vascular dementia is dementia caused by a series of strokes. Restricted blood flow due to strokes reduces oxygen and glucose delivery to the brain, causing cell injury and neurological deficits in the affected region. Subtypes of vascular dementia include subcortical vascular dementia, multi-infarct dementia, stroke-related dementia, and mixed dementia.

Cerebrovascular disease includes a variety of medical conditions that affect the blood vessels of the brain and the cerebral circulation. Arteries supplying oxygen and nutrients to the brain are often damaged or deformed in these disorders. The most common presentation of cerebrovascular disease is an ischemic stroke or mini-stroke and sometimes a hemorrhagic stroke. Hypertension is the most important contributing risk factor for stroke and cerebrovascular diseases as it can change the structure of blood vessels and result in atherosclerosis. Atherosclerosis narrows blood vessels in the brain, resulting in decreased cerebral perfusion. Other risk factors that contribute to stroke include smoking and diabetes. Narrowed cerebral arteries can lead to ischemic stroke, but continually elevated blood pressure can also cause tearing of vessels, leading to a hemorrhagic stroke.

Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain. This typically causes impaired nerve function, increased pressure within the skull, and can eventually lead to direct compression of brain tissue and blood vessels. Symptoms vary based on the location and extent of edema and generally include headaches, nausea, vomiting, seizures, drowsiness, visual disturbances, dizziness, and in severe cases, death.
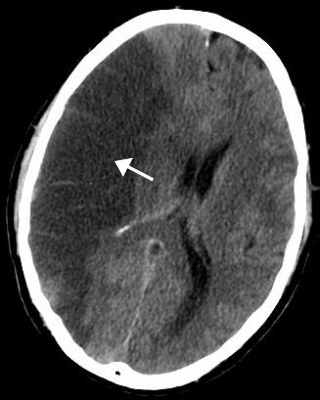
Stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly.

Cerebral hypoxia is a form of hypoxia, specifically involving the brain; when the brain is completely deprived of oxygen, it is called cerebral anoxia. There are four categories of cerebral hypoxia; they are, in order of increasing severity: diffuse cerebral hypoxia (DCH), focal cerebral ischemia, cerebral infarction, and global cerebral ischemia. Prolonged hypoxia induces neuronal cell death via apoptosis, resulting in a hypoxic brain injury.

Intracerebral hemorrhage (ICH), also known as hemorrhagic stroke, is a sudden bleeding into the tissues of the brain, into its ventricles, or into both. An ICH is a type of bleeding within the skull and one kind of stroke. Symptoms can vary dramatically depending on the severity, acuity, and location (anatomically) but can include headache, one-sided weakness, numbness, tingling, or paralysis, speech problems, vision or hearing problems, memory loss, attention problems, coordination problems, balance problems, dizziness or lightheadedness or vertigo, nausea/vomiting, seizures, decreased level of consciousness or total loss of consciousness, neck stiffness, and fever.

Cerebral infarction, also known as an ischemic stroke, is the pathologic process that results in an area of necrotic tissue in the brain. In mid to high income countries, a stroke is the main reason for disability among people and the 2nd cause of death. It is caused by disrupted blood supply (ischemia) and restricted oxygen supply (hypoxia). This is most commonly due to a thrombotic occlusion, or an embolic occlusion of major vessels which leads to a cerebral infarct. In response to ischemia, the brain degenerates by the process of liquefactive necrosis.

A watershed stroke is defined as a brain ischemia that is localized to the vulnerable border zones between the tissues supplied by the anterior, posterior and middle cerebral arteries. The actual blood stream blockage/restriction site can be located far away from the infarcts. Watershed locations are those border-zone regions in the brain supplied by the major cerebral arteries where blood supply is decreased. Watershed strokes are a concern because they comprise approximately 10% of all ischemic stroke cases. The watershed zones themselves are particularly susceptible to infarction from global ischemia as the distal nature of the vasculature predisposes these areas to be most sensitive to profound hypoperfusion.
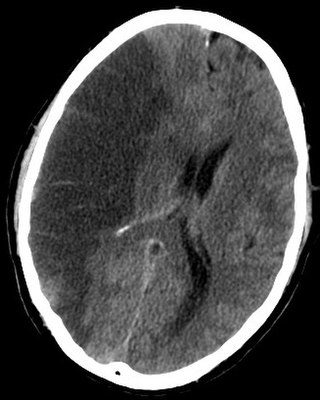
Brain ischemia is a condition in which there is insufficient bloodflow to the brain to meet metabolic demand. This leads to poor oxygen supply or cerebral hypoxia and thus leads to the death of brain tissue or cerebral infarction/ischemic stroke. It is a sub-type of stroke along with subarachnoid hemorrhage and intracerebral hemorrhage.
Animal models of ischemic stroke are procedures inducing cerebral ischemia. The aim is the study of basic processes or potential therapeutic interventions in this disease, and the extension of the pathophysiological knowledge on and/or the improvement of medical treatment of human ischemic stroke. Ischemic stroke has a complex pathophysiology involving the interplay of many different cells and tissues such as neurons, glia, endothelium, and the immune system. These events cannot be mimicked satisfactorily in vitro yet. Thus a large portion of stroke research is conducted on animals.
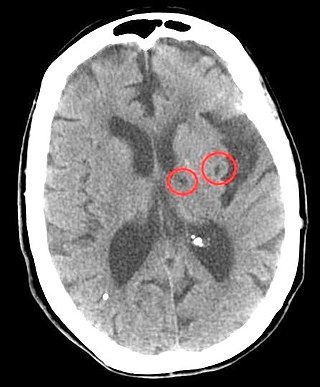
Lacunar stroke or lacunar cerebral infarct (LACI) is the most common type of ischemic stroke, resulting from the occlusion of small penetrating arteries that provide blood to the brain's deep structures. Patients who present with symptoms of a lacunar stroke, but who have not yet had diagnostic imaging performed, may be described as having lacunar stroke syndrome (LACS).
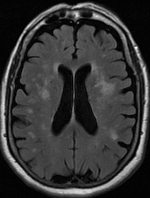
Leukoaraiosis is a particular abnormal change in appearance of white matter near the lateral ventricles. It is often seen in aged individuals, but sometimes in young adults. On MRI, leukoaraiosis changes appear as white matter hyperintensities (WMHs) in T2 FLAIR images. On CT scans, leukoaraiosis appears as hypodense periventricular white-matter lesions.

Cerebral venous sinus thrombosis (CVST), cerebral venous and sinus thrombosis or cerebral venous thrombosis (CVT), is the presence of a blood clot in the dural venous sinuses, the cerebral veins, or both. Symptoms may include severe headache, visual symptoms, any of the symptoms of stroke such as weakness of the face and limbs on one side of the body, and seizures, which occur in around 40% of patients.
In pathology and anatomy the penumbra is the area surrounding an ischemic event such as thrombotic or embolic stroke. Immediately following the event, blood flow and therefore oxygen transport is reduced locally, leading to hypoxia of the cells near the location of the original insult. This can lead to hypoxic cell death (infarction) and amplify the original damage from the ischemia; however, the penumbra area may remain viable for several hours after an ischemic event due to the collateral arteries that supply the penumbral zone.
Remote ischemic conditioning (RIC) is an experimental medical procedure that aims to reduce the severity of ischaemic injury to an organ such as the heart or the brain, most commonly in the situation of a heart attack or a stroke, or during procedures such as heart surgery when the heart may temporary suffer ischaemia during the operation, by triggering the body's natural protection against tissue injury. Although noted to have some benefits in experimental models in animals, this is still an experimental procedure in humans and initial evidence from small studies have not been replicated in larger clinical trials. Successive clinical trials have failed to identify evidence supporting a protective role in humans.
A migrainous infarction is a rare type of ischaemic stroke which occurs in correspondence with migraine aura symptoms. Symptoms include headaches, visual disturbances, strange sensations and dysphasia, all of which gradually worsen causing neurological changes which ultimately increase the risk of an ischaemic stroke. Typically, women under the age of 45 who experience migraine with aura (MA) are at the greatest risk for developing migrainous infarction, especially when combined with smoking and use of oral contraceptives.

Hypertension is a condition characterized by an elevated blood pressure in which the long term consequences include cardiovascular disease, kidney disease, adrenal gland tumors, vision impairment, memory loss, metabolic syndrome, stroke and dementia. It affects nearly 1 in 2 Americans and remains as a contributing cause of death in the United States. There are many genetic and environmental factors involved with the development of hypertension including genetics, diet, and stress.
A cerebroprotectant is a drug that is intended to protect the brain after the onset of acute ischemic stroke. As stroke is the second largest cause of death worldwide and a leading cause of adult disability, over 150 drugs have been tested in clinical trials to provide cerebroprotection.
Hemorrhagic transformation (HT) or hemorrhagic conversion is a medical complication that can occur in the brain following an acute ischemic stroke, a condition in which blood flow to the brain is blocked.
References
- ↑ Miwa, K; Hoshi, T; Hougaku, H; Tanaka, Makiko; Furukado, Shigetaka; Abe, Yuko; Okazaki, Shuhei; Sakaguchi, Manabu; et al. (2010). "Silent cerebral infarction is associated with incident stroke and TIA independent of carotid intima-media thickness". Internal Medicine (Tokyo, Japan). 49 (9): 817–22. doi: 10.2169/internalmedicine.49.3211 . PMID 20453400.
- 1 2 Herderscheê, D; Hijdra, A; Algra, A; Koudstaal, PJ; Kappelle, LJ; Van Gijn, J (1992). "Silent stroke in patients with transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group". Stroke: A Journal of Cerebral Circulation. 23 (9): 1220–4. doi: 10.1161/01.STR.23.9.1220 . PMID 1519274.
- ↑ Leary, MC; Saver, JL (2003). "Annual incidence of first silent stroke in the United States: A preliminary estimate". Cerebrovascular Diseases. 16 (3): 280–5. doi:10.1159/000071128. PMID 12865617. S2CID 33095581.
- ↑ Vermeer, SE; Koudstaal, PJ; Oudkerk, M; Hofman, A; Breteler, MM (2002). "Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study". Stroke: A Journal of Cerebral Circulation. 33 (1): 21–5. doi: 10.1161/hs0102.101629 . PMID 11779883.
- ↑ Yatsu, FM; Shaltoni, HM (2004). "Implications of silent strokes". Current Atherosclerosis Reports. 6 (4): 307–13. doi:10.1007/s11883-004-0063-0. PMID 15191706. S2CID 19688830.
- 1 2 Schmidt, WP; Roesler, A; Kretzschmar, K; Ladwig, KH; Junker, R; Berger, K (2004). "Functional and cognitive consequences of silent stroke discovered using brain magnetic resonance imaging in an elderly population". Journal of the American Geriatrics Society. 52 (7): 1045–50. doi: 10.1111/j.1532-5415.2004.52300.x . PMID 15209640. S2CID 19351339.
- ↑ Fried, LP; Borhani, NO; Enright, P; Furberg, CD; Gardin, JM; Kronmal, RA; Kuller, LH; Manolio, TA; et al. (1991). "The Cardiovascular Health Study: Design and rationale". Annals of Epidemiology. 1 (3): 263–76. doi:10.1016/1047-2797(91)90005-W. PMID 1669507.
- ↑ Coutts, SB; Hill, MD; Simon, JE; Sohn, CH; Scott, JN; Demchuk, AM; Vision Study, Group (2005). "Silent ischemia in minor stroke and TIA patients identified on MR imaging". Neurology. 65 (4): 513–7. doi:10.1212/01.WNL.0000169031.39264.ff. PMID 16116107. S2CID 24762370.
- ↑ Van Zagten, M; Boiten, J; Kessels, F; Lodder, J (1996). "Significant progression of white matter lesions and small deep (lacunar) infarcts in patients with stroke". Archives of Neurology. 53 (7): 650–5. doi:10.1001/archneur.1996.00550070088015. PMID 8929172.
- ↑ Igarashi, K; Kashiwagi, K (2011). "Use of Polyamine Metabolites as Markers for Stroke and Renal Failure". Polyamines. Methods in Molecular Biology. Vol. 720. pp. 395–408. doi:10.1007/978-1-61779-034-8_25. ISBN 978-1-61779-033-1. PMID 21318888.
- ↑ Tomitori, H; Usui, T; Saeki, N; Ueda, S; Kase, H; Nishimura, K; Kashiwagi, K; Igarashi, K (2005). "Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke". Stroke: A Journal of Cerebral Circulation. 36 (12): 2609–13. doi: 10.1161/01.STR.0000190004.36793.2d . PMID 16269634.
- ↑ Bang, OY; Saver, JL; Ovbiagele, B; Choi, YJ; Yoon, SR; Lee, KH (2007). "Adiponectin levels in patients with intracranial atherosclerosis". Neurology. 68 (22): 1931–7. doi:10.1212/01.wnl.0000263186.20988.9f. PMID 17536050. S2CID 41415046.
- ↑ Lim, JS; Kwon, HM (2010). "Risk of "silent stroke" in patients older than 60 years: Risk assessment and clinical perspectives". Clinical Interventions in Aging. 5: 239–51. doi: 10.2147/cia.s7382 . PMC 2938031 . PMID 20852671.
- ↑ Dowling, Michael M.; Quinn, Charles T.; Rogers, Zora R.; Buchanan, George R. (2010). "Acute silent cerebral infarction in children with sickle cell anemia". Pediatric Blood & Cancer. 54 (3): 461–4. doi:10.1002/pbc.22242. PMC 2807470 . PMID 19813251.
- ↑ Adams, RJ (2007). "Big strokes in small persons". Archives of Neurology. 64 (11): 1567–74. doi: 10.1001/archneur.64.11.1567 . PMID 17998439.
- ↑ Bernaudin, F; Verlhac, S; Fréard, F; Roudot-Thoraval, F; Benkerrou, M; Thuret, I; Mardini, R; Vannier, JP; et al. (2000). "Multicenter prospective study of children with sickle cell disease: Radiographic and psychometric correlation". Journal of Child Neurology. 15 (5): 333–43. doi:10.1177/088307380001500510. PMID 10830200. S2CID 25470082.
- ↑ Adams, RJ; Ohene-Frempong, K; Wang, W (2001). "Sickle cell and the brain". Hematology. 2001: 31–46. doi: 10.1182/asheducation-2001.1.31 . PMID 11722977.
- ↑ King, AA; Debaun, MR; White, DA (2008). "Need for cognitive rehabilitation for children with sickle cell disease and strokes". Expert Review of Neurotherapeutics. 8 (2): 291–6. doi:10.1586/14737175.8.2.291. PMID 18271713. S2CID 43723218.
- ↑ Caksen, H; Odabaş, D; Akbayram, S; Faik Oner, A; Arslan, S; Cesur, Y; Uner, A (2003). "Silent stroke in a case of beta-thalassemia major associated with chronic renal failure and diabetes mellitus". Journal of Child Neurology. 18 (11): 798–800. doi:10.1177/08830738030180110201. PMID 14696909. S2CID 28433612.
- ↑ Kalantarian S, Ay H, Gollub RL, Lee H, Retzepi K, Mansour M, Ruskin JN (4 Nov 2014). "Association Between Atrial Fibrillation and Silent Cerebral Infarctions: A Systematic Review and Meta-analysis". Ann Intern Med. 161 (9): 650–8. doi:10.7326/M14-0538. PMC 5578742 . PMID 25364886.
- ↑ Li, M; Yu, D; Williams, KJ; Liu, ML (2010). "Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages". Arteriosclerosis, Thrombosis, and Vascular Biology. 30 (9): 1818–24. doi:10.1161/ATVBAHA.110.209577. PMC 2939448 . PMID 20558816.
- ↑ Mazzone, P; Tierney, W; Hossain, M; Puvenna, V; Janigro, D; Cucullo, L (2010). "Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood–brain barrier: Expanding the awareness of smoking toxicity in an underappreciated area". International Journal of Environmental Research and Public Health. 7 (12): 4111–26. doi: 10.3390/ijerph7124111 . PMC 3037043 . PMID 21317997.
- ↑ Lind, L; Sarabi, M; Millgård, J (2003). "The effect of smoking on endothelial vasodilatory function evaluated by local infusion of metacholine in the forearm is dependent on the duration of smoking". Nicotine & Tobacco Research. 5 (1): 125–30. doi:10.1080/1462220031000070516. PMID 12745514.
- ↑ Kubota, K; Yamaguchi, T; Abe, Y; Fujiwara, T; Hatazawa, J; Matsuzawa, T (1983). "Effects of smoking on regional cerebral blood flow in neurologically normal subjects". Stroke: A Journal of Cerebral Circulation. 14 (5): 720–4. doi: 10.1161/01.STR.14.5.720 . PMID 6658956.
- ↑ Love, BB; Biller, J; Jones, MP; Adams Jr, HP; Bruno, A (1990). "Cigarette smoking. A risk factor for cerebral infarction in young adults". Archives of Neurology. 47 (6): 693–8. doi:10.1001/archneur.1990.00530060107027. PMID 2189378.
- ↑ Ridker PM, Libby P (2007). "Risk Factors for Atherothrombotic Disease". In Libby P, Bonow RO, Mann DL, Zipes DP (eds.). Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (8th ed.). Philadelphia: Saunders Elsevier. pp. 939–.
- ↑ Yoshida, M; Tomitori, H; Machi, Y; Katagiri, D; Ueda, S; Horiguchi, K; Kobayashi, E; Saeki, N; et al. (2009). "Acrolein, IL-6 and CRP as markers of silent brain infarction". Atherosclerosis. 203 (2): 557–62. doi:10.1016/j.atherosclerosis.2008.07.022. PMID 18757054.
- ↑ Roberts, RO; Kantarci, K; Geda, YE; Knopman, DS; Przybelski, SA; Weigand, SD; Petersen, RC; Jack Jr, CR (2011). "Untreated type 2 diabetes and its complications are associated with subcortical infarctions". Diabetes Care. 34 (1): 184–6. doi:10.2337/dc10-0602. PMC 3005470 . PMID 20980413.
- ↑ Chen, X; Wen, W; Anstey, KJ; Sachdev, PS (2009). "Prevalence, incidence, and risk factors of lacunar infarcts in a community sample". Neurology. 73 (4): 266–72. doi:10.1212/WNL.0b013e3181aa52ea. hdl: 1959.4/unsworks_40331 . PMID 19636046. S2CID 25658551.
- ↑ Vermeer, SE; Van Dijk, EJ; Koudstaal, PJ; Oudkerk, M; Hofman, A; Clarke, R; Breteler, MM (2002). "Homocysteine, silent brain infarcts, and white matter lesions: The Rotterdam Scan Study". Annals of Neurology. 51 (3): 285–9. doi:10.1002/ana.10111. PMID 11891822. S2CID 7860349.
- ↑ Seshadri, S; Wolf, PA; Beiser, AS; Selhub, J; Au, R; Jacques, PF; Yoshita, M; Rosenberg, IH; et al. (2008). "Association of plasma total homocysteine levels with subclinical brain injury: Cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study". Archives of Neurology. 65 (5): 642–9. doi:10.1001/archneur.65.5.642. PMC 2700952 . PMID 18474741.
- ↑ Matsui, T; Arai, H; Yuzuriha, T; Yao, H; Miura, M; Hashimoto, S; Higuchi, S; Matsushita, S; et al. (2001). "Elevated plasma homocysteine levels and risk of silent brain infarction in elderly people". Stroke: A Journal of Cerebral Circulation. 32 (5): 1116–9. doi: 10.1161/01.STR.32.5.1116 . PMID 11340219.
- ↑ Bokura, H; Yamaguchi, S; Iijima, K; Nagai, A; Oguro, H (2008). "Metabolic syndrome is associated with silent ischemic brain lesions". Stroke: A Journal of Cerebral Circulation. 39 (5): 1607–9. doi: 10.1161/STROKEAHA.107.508630 . PMID 18323475.
- ↑ Kwon, HM; Kim, BJ; Park, JH; Ryu, WS; Kim, CK; Lee, SH; Ko, SB; Nam, H; et al. (2009). "Significant association of metabolic syndrome with silent brain infarction in elderly people". Journal of Neurology. 256 (11): 1825–31. doi:10.1007/s00415-009-5201-8. PMID 19533202. S2CID 28634392.
- ↑ De Groot, PC; Dekkers, OM; Romijn, JA; Dieben, SW; Helmerhorst, FM (2011). "PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis". Human Reproduction Update. 17 (4): 495–500. doi: 10.1093/humupd/dmr001 . PMID 21335359.
- ↑ Chokroverty S (February 2010). "Overview of sleep & sleep disorders". Indian J. Med. Res. 131: 126–40. PMID 20308738.
- ↑ American Stroke Association Meeting Report - Abstract 3434/P161: Sleep apnea linked to silent strokes, small lesions in brain "Sleep apnea linked to silent strokes, small lesions in brain / American Heart Association". Archived from the original on 2012-02-11. Retrieved 2012-02-05.
- ↑ Price TR, Manolio TA, Kronmal RA, et al. (June 1997). "Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group". Stroke. 28 (6): 1158–64. doi:10.1161/01.STR.28.6.1158. PMID 9183343.
- ↑ Maeshima S, Moriwaki H, Ozaki F, Okita R, Yamaga H, Ueyoshi A (March 2002). "Silent cerebral infarction and cognitive function in middle-aged neurologically healthy subjects". Acta Neurol. Scand. 105 (3): 179–84. doi: 10.1034/j.1600-0404.2002.1o068.x . PMID 11886361. S2CID 40398490.
- ↑ Pueyo R, Junqué C, Vendrell P, Narberhaus A, Segarra D (May 2008). "Raven's Coloured Progressive Matrices as a measure of cognitive functioning in Cerebral Palsy". Journal of Intellectual Disability Research. 52 (Pt 5): 437–45. doi:10.1111/j.1365-2788.2008.01045.x. PMID 18312310.
- ↑ Sam Goldstein, Cecil R. Reynolds: Handbook of Neurodevelopmental and Genetic Disorders in Children p.105 (2010) ISBN 1-60623-990-2
- ↑ Kral MC, Brown RT, Hynd GW (December 2001). "Neuropsychological aspects of pediatric sickle cell disease". Neuropsychol Rev. 11 (4): 179–96. doi:10.1023/A:1012901124088. PMID 11883668. S2CID 10970844.
- ↑ Fujikawa, T; Yamawaki, S; Touhouda, Y (1994). "Background factors and clinical symptoms of major depression with silent cerebral infarction". Stroke: A Journal of Cerebral Circulation. 25 (4): 798–801. doi: 10.1161/01.STR.25.4.798 . PMID 8160223.
- ↑ Price, TR; Manolio, TA; Kronmal, RA; Kittner, SJ; Yue, NC; Robbins, J; Anton-Culver, H; O'Leary, DH (1997). "Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group". Stroke: A Journal of Cerebral Circulation. 28 (6): 1158–64. doi:10.1161/01.STR.28.6.1158. PMID 9183343.
- ↑ Vermeer, SE; Longstreth Jr, WT; Koudstaal, PJ (2007). "Silent brain infarcts: A systematic review". Lancet Neurology. 6 (7): 611–9. doi:10.1016/S1474-4422(07)70170-9. PMID 17582361. S2CID 11286803.
- ↑ Corea, F; Tambasco, N; Luccioli, R; Ciorba, E; Parnetti, L; Gallai, V (2002). "Brain CT-scan in acute stroke patients: Silent infarcts and relation to outcome". Clinical and Experimental Hypertension. 24 (7–8): 669–76. doi:10.1081/CEH-120015343. PMID 12450242. S2CID 5972382.
- ↑ Boon, A; Lodder, J; Heuts-Van Raak, L; Kessels, F (1994). "Silent brain infarcts in 755 consecutive patients with a first-ever supratentorial ischemic stroke. Relationship with index-stroke subtype, vascular risk factors, and mortality". Stroke: A Journal of Cerebral Circulation. 25 (12): 2384–90. doi: 10.1161/01.STR.25.12.2384 . PMID 7974577.
- ↑ Ong, CT; Chen, WP; Sung, SF; Wu, CS; Hsu, YC (2007). "Silent infarction in patients with first-ever stroke". Acta Neurologica Taiwanica. 16 (4): 221–5. PMID 18220015.
- ↑ Adams, R; McKie, V; Nichols, F; Carl, E; Zhang, DL; McKie, K; Figueroa, R; Litaker, M; et al. (1992). "The use of transcranial ultrasonography to predict stroke in sickle cell disease". The New England Journal of Medicine. 326 (9): 605–10. doi: 10.1056/NEJM199202273260905 . PMID 1734251.
- ↑ Pegelow, CH; Wang, W; Granger, S; Hsu, LL; Vichinsky, E; Moser, FG; Bello, J; Zimmerman, RA; et al. (2001). "Silent infarcts in children with sickle cell anemia and abnormal cerebral artery velocity". Archives of Neurology. 58 (12): 2017–21. doi: 10.1001/archneur.58.12.2017 . PMID 11735775.
- ↑ Lee, MT; Piomelli, S; Granger, S; Miller, ST; Harkness, S; Brambilla, DJ; Adams, RJ; Stop Study, Investigators (2006). "Stroke Prevention Trial in Sickle Cell Anemia (STOP): Extended follow-up and final results". Blood. 108 (3): 847–52. doi:10.1182/blood-2005-10-009506. PMC 1895848 . PMID 16861341.