Silicate perovskite is either (Mg,Fe)SiO3 (the magnesium end-member is called bridgmanite [1] ) or CaSiO3 (calcium silicate known as davemaoite) when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the lower part of Earth's mantle, between about 670 and 2,700 km (420 and 1,680 mi) depth. They are thought to form the main mineral phases of the lower mantle, together with ferropericlase.
The existence of silicate perovskite in the mantle was first suggested in 1962, and both MgSiO3 and CaSiO3 had been synthesized experimentally before 1975. By the late 1970s, it had been proposed that the seismic discontinuity at about 660 km in the mantle represented a change from spinel structure minerals with an olivine composition to silicate perovskite with ferropericlase.
Natural silicate perovskite was discovered in the heavily shocked Tenham meteorite. [2] [3] In 2014, the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) approved the name bridgmanite for perovskite-structured (Mg,Fe)SiO3, [1] in honor of physicist Percy Bridgman, who was awarded the Nobel Prize in Physics in 1946 for his high-pressure research. [4]
In 2021 perovskite-structured CaSiO3 was found as an inclusion in a natural diamond. The name davemaoite has been adopted for this mineral. [5]
The perovskite structure (first identified in the mineral perovskite) occurs in substances with the general formula ABX3, where A is a metal that forms large cations, typically magnesium, ferrous iron, or calcium. B is another metal that forms smaller cations, typically silicon, although minor amounts of ferric iron and aluminum can occur. X is typically oxygen. The structure may be cubic, but only if the relative sizes of the ions meet strict criteria. Typically, substances with the perovskite structure show lower symmetry, owing to the distortion of the crystal lattice and silicate perovskites are in the orthorhombic crystal system. [6]
Bridgmanite is a high-pressure polymorph of enstatite, but in the Earth predominantly forms, along with ferropericlase, from the decomposition of ringwoodite (a high-pressure form of olivine) at approximately 660 km depth, or a pressure of about 24 GPa. [6] [7] The depth of this transition depends on the mantle temperature; it occurs slightly deeper in colder regions of the mantle and shallower in warmer regions. [8] The transition from ringwoodite to bridgmanite and ferropericlase marks the bottom of the mantle transition zone and the top of the lower mantle. Bridgmanite becomes unstable at a depth of approximately 2700 km, transforming isochemically to post-perovskite. [9]
Calcium silicate perovskite is stable at slightly shallower depths than bridgmanite, becoming stable at approximately 500 km, and remains stable throughout the lower mantle. [9]
Bridgmanite is the most abundant mineral in the mantle. The proportions of bridgmanite and calcium perovskite depends on the overall lithology and bulk composition. In pyrolitic and harzburgitic lithogies, bridgmanite constitutes around 80% of the mineral assemblage, and calcium perovskite less than 10%. In an eclogitic lithology, bridgmanite and calcium perovskite comprise about 30% each. [9] Magnesium silicate perovskite is probably the most abundant mineral phase in the Earth. [3]
Calcium silicate perovskite has been identified at Earth's surface as inclusions in diamonds. [10] The diamonds are formed under high pressure deep in the mantle. With the great mechanical strength of the diamonds a large part of this pressure is retained inside the lattice, enabling inclusions such as the calcium silicate to be preserved in high-pressure form.
Experimental deformation of polycrystalline MgSiO3 under the conditions of the uppermost part of the lower mantle suggests that silicate perovskite deforms by a dislocation creep mechanism. This may help explain the observed seismic anisotropy in the mantle. [11]

The mineral olivine is a magnesium iron silicate with the chemical formula (Mg,Fe)2SiO4. It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. Olivine has many uses, such as the gemstone peridot, as well as industrial applications like metalworking processes.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where both/either the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Periclase is a magnesium mineral that occurs naturally in contact metamorphic rocks and is a major component of most basic refractory bricks. It is a cubic form of magnesium oxide (MgO). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).
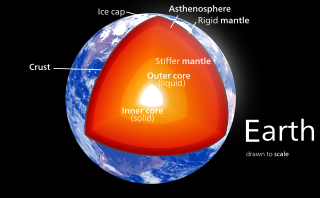
Earth's mantle is a layer of silicate rock between the crust and the outer core. It has a mass of 4.01×1024 kg (8.84×1024 lb) and makes up 67% of the mass of Earth. It has a thickness of 2,900 kilometers (1,800 mi) making up about 46% of Earth's radius and 84% of Earth's volume. It is predominantly solid but, on geologic time scales, it behaves as a viscous fluid, sometimes described as having the consistency of caramel. Partial melting of the mantle at mid-ocean ridges produces oceanic crust, and partial melting of the mantle at subduction zones produces continental crust.
Post-perovskite (pPv) is a high-pressure phase of magnesium silicate (MgSiO3). It is composed of the prime oxide constituents of the Earth's rocky mantle (MgO and SiO2), and its pressure and temperature for stability imply that it is likely to occur in portions of the lowermost few hundred km of Earth's mantle.

Wadsleyite is an orthorhombic mineral with the formula β-(Mg,Fe)2SiO4. It was first found in nature in the Peace River meteorite from Alberta, Canada. It is formed by a phase transformation from olivine (α-(Mg,Fe)2SiO4) under increasing pressure and eventually transforms into spinel-structured ringwoodite (γ-(Mg,Fe)2SiO4) as pressure increases further. The structure can take up a limited amount of other bivalent cations instead of magnesium, but contrary to the α and γ structures, a β structure with the sum formula Fe2SiO4 is not thermodynamically stable. Its cell parameters are approximately a = 5.7 Å, b = 11.71 Å and c = 8.24 Å.

Ringwoodite is a high-pressure phase of Mg2SiO4 (magnesium silicate) formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It may also contain iron and hydrogen. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate).

Majorite is a mineral found in the mantle of the Earth. Its chemical formula is Mg3(MgSi)(SiO4)3. It is a type of garnet, distinguished from other garnets in having silicon in octahedral as well as tetrahedral coordination. Majorite was first described in 1970 from the Coorara Meteorite of Western Australia and has been reported from various other meteorites in which majorite is thought to result from an extraterrestrial high pressure shock event. Mantle-derived xenoliths containing majorite have been reported from potassic ultramafic magmas on Malaita Island on the Ontong Java Plateau in the southwest Pacific Ocean.
Ferropericlase or magnesiowüstite is a magnesium/iron oxide with the chemical formula (Mg,Fe)O that is interpreted to be one of the main constituents of the Earth's lower mantle together with the silicate perovskite, a magnesium/iron silicate with a perovskite structure. Ferropericlase has been found as inclusions in a few natural diamonds. An unusually high iron content in one suite of diamonds has been associated with an origin from the lowermost mantle. Discrete ultralow-velocity zones in the deepest parts of the mantle, near the Earth's core, are thought to be blobs of ferropericlase, as seismic waves are significantly slowed as they pass through them, and ferropericlase is known to have this effect at the high pressures and temperatures found deep within the Earth's mantle. In May 2018, ferropericlase was shown to be anisotropic in specific ways in the high pressures of the lower mantle, and these anisotropies may help seismologists and geologists to confirm whether those ultra-low velocity zones are indeed ferropericlase, by passing seismic waves through them from various different directions and observing the exact amount of change in the velocity of those waves.
Akimotoite is a rare silicate mineral in the ilmenite group of minerals, with the chemical formula (Mg,Fe)SiO3. It is polymorphous with pyroxene and with bridgmanite, a natural silicate perovskite that is the most abundant mineral in Earth's silicate mantle. Akimotoite has a vitreous luster, is colorless, and has a white or colorless streak. It crystallizes in the trigonal crystal system in space group R3. It is the silicon analogue of geikielite (MgTiO3).

Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula CaTiO3). Its name is also applied to the class of compounds which have the same type of crystal structure as CaTiO3, known as the perovskite structure, which has a general chemical formula A2+B4+(X2−)3. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials.

Tenham meteorites are the fragments of a larger meteorite that fell in 1879 in a remote area of Australia near the Tenham station, South Gregory, in western Queensland. Although the fall was seen by a number of people, its exact date has not been established. Bright meteors were seen to be moving roughly from west to east. Stones were subsequently recovered from over a large area, about 20 kilometres (12 mi) long by 5 kilometres (3.1 mi) wide.

In chemistry, germanate is a compound containing an oxyanion of germanium. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central germanium atom, for example potassium hexafluorogermanate, K2GeF6.

Mantle oxidation state (redox state) applies the concept of oxidation state in chemistry to the study of the Earth's mantle. The chemical concept of oxidation state mainly refers to the valence state of one element, while mantle oxidation state provides the degree of decreasing of increasing valence states of all polyvalent elements in mantle materials confined in a closed system. The mantle oxidation state is controlled by oxygen fugacity and can be benchmarked by specific groups of redox buffers.

The lower mantle, historically also known as the mesosphere, represents approximately 56% of Earth's total volume, and is the region from 660 to 2900 km below Earth's surface; between the transition zone and the outer core. The preliminary reference Earth model (PREM) separates the lower mantle into three sections, the uppermost (660–770 km), mid-lower mantle (770–2700 km), and the D layer (2700–2900 km). Pressure and temperature in the lower mantle range from 24–127 GPa and 1900–2600 K. It has been proposed that the composition of the lower mantle is pyrolitic, containing three major phases of bridgmanite, ferropericlase, and calcium-silicate perovskite. The high pressure in the lower mantle has been shown to induce a spin transition of iron-bearing bridgmanite and ferropericlase, which may affect both mantle plume dynamics and lower mantle chemistry.
The upper mantle of Earth is a very thick layer of rock inside the planet, which begins just beneath the crust and ends at the top of the lower mantle at 670 km (420 mi). Temperatures range from approximately 500 K at the upper boundary with the crust to approximately 1,200 K at the boundary with the lower mantle. Upper mantle material that has come up onto the surface comprises about 55% olivine, 35% pyroxene, and 5 to 10% of calcium oxide and aluminum oxide minerals such as plagioclase, spinel, or garnet, depending upon depth.

Davemaoite is a high-pressure calcium silicate perovskite (CaSiO3) mineral with a distinctive cubic crystal structure. It is named after geophysicist Ho-kwang (Dave) Mao, who pioneered in many discoveries in high-pressure geochemistry and geophysics.

Diamond inclusions are the non-diamond materials that get encapsulated inside diamond during its formation process in the mantle. The trapped materials can be other minerals or fluids like water. Since diamonds have high strength and low reactivity with either the inclusion or the volcanic host rocks which carry the diamond to the Earth's surface, the diamond serves as a container that preserves the included material intact under the changing conditions from the mantle to the surface. Although diamonds can only place a lower bound on the pressure of their formation, many inclusions provide additional constraints on the pressure, temperature and even age of formation.
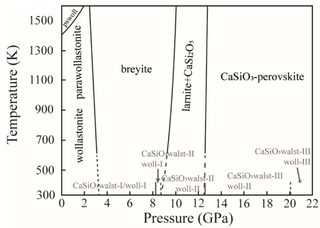
Breyite is a high pressure calcium silicate mineral (CaSiO3) found in diamond inclusions. It is the second most abundant inclusion after ferropericlase, for diamonds with a deep Earth origin. Its occurrence can also indicate the host diamond's super-deep origin. This mineral is named after German mineralogist, petrologist and geochemist Gerhard P. Brey.