Related Research Articles

A circadian rhythm, or circadian cycle, is a natural oscillation that repeats roughly every 24 hours. Circadian rhythms can refer to any process that originates within an organism and responds to the environment. Circadian rhythms are regulated by a circadian clock whose primary function is to rhythmically co-ordinate biological processes so they occur at the correct time to maximise the fitness of an individual. Circadian rhythms have been widely observed in animals, plants, fungi and cyanobacteria and there is evidence that they evolved independently in each of these kingdoms of life.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.
A circadian clock, or circadian oscillator, is a biochemical oscillator that cycles with a stable phase and is synchronized with solar time.
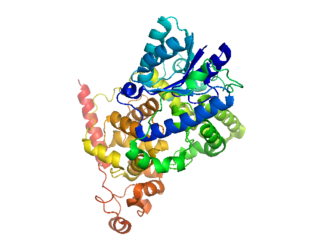
Cryptochromes are a class of flavoproteins found in plants and animals that are sensitive to blue light. They are involved in the circadian rhythms and the sensing of magnetic fields in a number of species. The name cryptochrome was proposed as a portmanteau combining the chromatic nature of the photoreceptor, and the cryptogamic organisms on which many blue-light studies were carried out.
The repressilator is a genetic regulatory network consisting of at least one feedback loop with at least three genes, each expressing a protein that represses the next gene in the loop. In biological research, repressilators have been used to build cellular models and understand cell function. There are both artificial and naturally-occurring repressilators. Recently, the naturally-occurring repressilator clock gene circuit in Arabidopsis thaliana and mammalian systems have been studied.
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals.

Michael Morris Rosbash is an American geneticist and chronobiologist. Rosbash is a professor and researcher at Brandeis University and investigator at the Howard Hughes Medical Institute. Rosbash's research group cloned the Drosophila period gene in 1984 and proposed the Transcription Translation Negative Feedback Loop for circadian clocks in 1990. In 1998, they discovered the cycle gene, clock gene, and cryptochrome photoreceptor in Drosophila through the use of forward genetics, by first identifying the phenotype of a mutant and then determining the genetics behind the mutation. Rosbash was elected to the National Academy of Sciences in 2003. Along with Michael W. Young and Jeffrey C. Hall, he was awarded the 2017 Nobel Prize in Physiology or Medicine "for their discoveries of molecular mechanisms controlling the circadian rhythm".
John B. Hogenesch is an American chronobiologist and Professor of Pediatrics at the Cincinnati Children's Hospital Medical Center. The primary focus of his work has been studying the network of mammalian clock genes from the genomic and computational perspective to further the understanding of circadian behavior. He is currently the Deputy Director of the Center for Chronobiology, an Ohio Eminent Scholar, and Professor of Pediatrics in the Divisions of Perinatal Biology and Immunobiology at the Cincinnati Children's Hospital Medical Center.
Circadian Clock Associated 1 (CCA1) is a gene that is central to the circadian oscillator of angiosperms. It was first identified in Arabidopsis thaliana in 1993. CCA1 interacts with LHY and TOC1 to form the core of the oscillator system. CCA1 expression peaks at dawn. Loss of CCA1 function leads to a shortened period in the expression of many other genes.
LUX or Phytoclock1 (PCL1) is a gene that codes for LUX ARRHYTHMO, a protein necessary for circadian rhythms in Arabidopsis thaliana. LUX protein associates with Early Flowering 3 (ELF3) and Early Flowering 4 (ELF4) to form the Evening Complex (EC), a core component of the Arabidopsis repressilator model of the plant circadian clock. The LUX protein functions as a transcription factor that negatively regulates Pseudo-Response Regulator 9 (PRR9), a core gene of the Midday Complex, another component of the Arabidopsis repressilator model. LUX is also associated with circadian control of hypocotyl growth factor genes PHYTOCHROME INTERACTING FACTOR 4 (PIF4) and PHYTOCHROME INTERACTING FACTOR 5 (PIF5).
Pseudo-response regulator (PRR) refers to a group of genes that regulate the circadian oscillator in plants. There are four primary PRR proteins that perform the majority of interactions with other proteins within the circadian oscillator, and another (PRR3) that has limited function. These genes are all paralogs of each other, and all repress the transcription of Circadian Clock Associated 1 (CCA1) and Late Elongated Hypocotyl (LHY) at various times throughout the day. The expression of PRR9, PRR7, PRR5 and TOC1/PRR1 peak around morning, mid-day, afternoon and evening, respectively. As a group, these genes are one part of the three-part repressilator system that governs the biological clock in plants.
The Late Elongated Hypocotyl gene (LHY), is an oscillating gene found in plants that functions as part of their circadian clock. LHY encodes components of mutually regulatory negative feedback loops with Circadian Clock Associated 1 (CCA1) in which overexpression of either results in dampening of both of their expression. This negative feedback loop affects the rhythmicity of multiple outputs creating a daytime protein complex. LHY was one of the first genes identified in the plant clock, along with TOC1 and CCA1. LHY and CCA1 have similar patterns of expression, which is capable of being induced by light. Single loss-of-function mutants in both genes result in seemingly identical phenotypes, but LHY cannot fully rescue the rhythm when CCA1 is absent, indicating that they may only be partially functionally redundant. Under constant light conditions, CCA1 and LHY double loss-of-function mutants fail to maintain rhythms in clock-controlled RNAs.
Transcription-translation feedback loop (TTFL) is a cellular model for explaining circadian rhythms in behavior and physiology. Widely conserved across species, the TTFL is auto-regulatory, in which transcription of clock genes is regulated by their own protein products.
Jay Dunlap is an American chronobiologist and photobiologist who has made significant contributions to the field of chronobiology by investigating the underlying mechanisms of circadian systems in Neurospora, a fungus commonly used as a model organism in biology, and in mice and mammalian cell culture models. Major contributions by Jay Dunlap include his work investigating the role of frq and wc clock genes in circadian rhythmicity, and his leadership in coordinating the whole genome knockout collection for Neurospora. He is currently the Nathan Smith Professor of Molecular and Systems Biology at the Geisel School of Medicine at Dartmouth. He and his colleague Jennifer Loros have mentored numerous students and postdoctoral fellows, many of whom presently hold positions at various academic institutions.
Carla Beth Green is an American neurobiologist and chronobiologist. She is a professor in the Department of Neuroscience and a Distinguished Scholar in Neuroscience at the University of Texas Southwestern Medical Center. She is the former president of the Society for Research on Biological Rhythms (SRBR), as well as a satellite member of the International Institute for Integrative Sleep Medicine at the University of Tsukuba in Japan.
Dmitri Nusinow is an American chronobiologist who studies plant circadian rhythms. He was born on November 7, 1976, in Inglewood, California. He currently resides in St. Louis, and his research focus includes a combination of molecular, biochemical, genetic, genomic, and proteomic tools to discover the molecular connections between signaling networks, circadian oscillators, and specific outputs. By combining these methods, he hopes to apply the knowledge elucidated from the Arabidopsis model to other plant species.
EARLY FLOWERING 3 (ELF3) is a plant-specific gene that encodes the hydroxyproline-rich glycoprotein and is required for the function of the circadian clock. ELF3 is one of the three components that make up the Evening Complex (EC) within the plant circadian clock, in which all three components reach peak gene expression and protein levels at dusk. ELF3 serves as a scaffold to bind EARLY FLOWERING 4 (ELF4) and LUX ARRHYTHMO (LUX), two other components of the EC, and functions to control photoperiod sensitivity in plants. ELF3 also plays an important role in temperature and light input within plants for circadian clock entrainment. Additionally, it plays roles in light and temperature signaling that are independent from its role in the EC.
Elaine Munsey Tobin is a professor of molecular, cell, and developmental biology at the University of California, Los Angeles (UCLA). Tobin is recognized as a Pioneer Member of the American Society of Plant Biologists (ASPB).
The chlorophyll a/b-binding protein gene, otherwise known as the CAB gene, is one of the most thoroughly characterized clock-regulated genes in plants. There are a variety of CAB proteins that are derived from this gene family. Studies on Arabidopsis plants have shed light on the mechanisms of biological clocks under the regulation of CAB genes. Dr. Steve Kay discovered that CAB was regulated by a circadian clock, which switched the gene on in the morning and off in the late afternoon. The genes code for proteins that associate with chlorophyll and xanthophylls. This association aids the absorption of sunlight, which transfers energy to photosystem II to drive photosynthetic electron transport.
Stacey Harmer is a chronobiologist whose work centers on the study of circadian rhythms in plants. Her research focuses on the molecular workings of the plant circadian clock and its influences on plant behaviors and physiology. She is a professor in the Department of Plant Biology at the University of California, Davis.
References
- ↑ Tripathi, Pratheek (2014). "Chronobiology: Past, Present and Future". ASPB Plant Science Blog.
- 1 2 "Scripps Research Institute Names Peter Schultz as CEO, Steve Kay as President".
- 1 2 3 4 5 6 7 8 9 Trivedi, Bijal (2009). "Profile of Steve Kay". PNAS. 106 (43): 18051–18053. Bibcode:2009PNAS..10618051T. doi: 10.1073/pnas.0910583106 . PMC 2775350 . PMID 19846773.
- ↑ Smieszek, Sandra (2014). "Steve Kay". ASPB News. 41 (2): 13.
- 1 2 3 4 Open Source Initiative Contributor. "Steve. A. Kay. Ph.D." Archived 2015-02-26 at the Wayback Machine . Retrieved on 08 April 2015.
- ↑ Fikes, Bradley J. "Scripps Research president returns to USC". sandiegouniontribune.com. Retrieved 29 September 2017.
- ↑ Atkins, K.A. & Dodd, A.N. (2014). "Circadian Regulation of Chloroplasts". Current Opinion in Plant Biology. 21: 43–50. doi:10.1016/j.pbi.2014.06.008. PMID 25026538.
- ↑ McClung, C.R. (2006). "Plant Circadian Rhythms". The Plant Cell. 18 (4): 792–803. doi:10.1105/tpc.106.040980. PMC 1425852 . PMID 16595397.
- ↑ Dunlap, J.C. (1999). "Molecular Bases for Circadian Clocks". Cell. 96 (2): 271–290. doi: 10.1016/S0092-8674(00)80566-8 . PMID 9988221. S2CID 14991100.
- ↑ Nagel, D. H. & Kay, S. A. (2012). "Complexity in the Wiring and Regulation of Plant Circadian Networks". Current Biology. 22 (16): 648–657. doi:10.1016/j.cub.2012.07.025. PMC 3427731 . PMID 22917516.
- ↑ Imaizumi, T. (2010). "Arabidopsis Circadian Clock and Photoperiodism:Time to Think about Location". Current Opinion in Plant Biology. 13 (1): 83–89. doi:10.1016/j.pbi.2009.09.007. PMC 2818179 . PMID 19836294.
- ↑ Pokhilko, A.; et al. (2012). "The Clock Gene Circuit in Arabidopsis Includes a Repressilator with Additional Feedback Loops". Molecular Systems Biology. 8: 574. doi:10.1038/msb.2012.6. PMC 3321525 . PMID 22395476.
- ↑ Boss, P. K., Bastow, R. M., Mylne, J. S. & Dean, C. (2004). "Multiple pathways in the decision to flower: enabling, promoting, and resetting". The Plant Cell. 16 (Suppl): S18–S31. doi:10.1105/tpc.015958. PMC 2643402 . PMID 15037730.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Chen, M., Chory, J. & Fankhauser, C. (2004). "Light signal transduction in higher plants". Annual Review of Genetics. 38: 87–117. doi:10.1146/annurev.genet.38.072902.092259. PMID 15568973.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Contag, C. H. & Bachmann, M. H. (2002). "Advances in In Vivo Bioluminescence Imaging of Gene Expression". Annual Review of Biomedical Engineering. 4: 235–260. doi:10.1146/annurev.bioeng.4.111901.093336. PMID 12117758.
- ↑ Hastings, M. H., Reddy, A. B. & Maywood, E. S. (2003). "A Clockwork Web: Circadian Timing in Brain and Periphery, in Health and Disease". Nature Reviews Neuroscience. 4 (8): 649–661. doi:10.1038/nrn1177. PMID 12894240. S2CID 205499642.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Young, M. W. & Kay, S. A. (2001). "Time Zones: A Comparative Genetics of Circadian Clocks". Nature Reviews Genetics. 2 (9): 702–715. doi:10.1038/35088576. PMID 11533719. S2CID 13286388.
- ↑ Hardin, P. E. (2005). "The Circadian Timekeeping System of Drosophila". Current Biology. 15 (17): 714–R722. doi: 10.1016/j.cub.2005.08.019 . PMID 16139204. S2CID 14904841.
- ↑ Satchidananda Panda, Ignacio Provencio, Daniel C. Tu, Susana S. Pires, Mark D. Rollag, Ana Maria Castrucci, Mathew T. Pletcher, Trey K. Sato, Tim Wiltshire, Mary Andahazy, Steve A. Kay, Russell N. Van Gelder and John B. Hogenesch (2003). "Melanopsin Is Required for Non-Image-Forming Photic Responses in Blind Mice". Science. 301 (5632): 525–527. Bibcode:2003Sci...301..525P. doi:10.1126/science.1086179. PMID 12829787. S2CID 37600812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Charna Dibner; Ueli Schibler & Urs Albrecht (2010). "The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks" (PDF). Annual Review of Physiology. 72: 517–549. doi:10.1146/annurev-physiol-021909-135821. PMID 20148687.
- ↑ Mario H. Bengtson & Claudio A.P. Joazeiro (2010). "Listerin-Dependent Nascent Protein Ubiquitination Relies on Ribosome Subunit Dissociation". Nature. 467 (7314): 470–473. doi:10.1038/nature09371. PMC 2988496 . PMID 20835226.
- ↑ Hirota T, et al. (2008). "A Chemical Biology Approach Reveals Period Shortening of the Mammalian Circadian Clock by Specific Inhibition of GSK-3β". Proceedings of the National Academy of Sciences USA. 105 (52): 20746–20751. Bibcode:2008PNAS..10520746H. doi: 10.1073/pnas.0811410106 . PMC 2606900 . PMID 19104043.
- ↑ Doherty, Colleen, Kay, Steve (2012). "Circadian Surprise- It's Not All About Transcription". Science. 338 (6105): 338–340. Bibcode:2012Sci...338..338D. doi:10.1126/science.1230008. PMID 23087238. S2CID 37498090.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Hirota T; Lee JW; St. John PC; Sawa M (2012). "Identification of small molecule activators in cryptochrome". Science. 337 (6098): 1094–1097. Bibcode:2012Sci...337.1094H. doi:10.1126/science.1223710. PMC 3589997 . PMID 22798407.
- ↑ Francis Lévi; Alper Okyar; Sandrine Dulong; Pasquale F. Innominato & Jean Clairambault (2010). "Circadian Timing in Cancer Treatments". Annual Review of Pharmacology and Toxicology. 50: 377–421. doi:10.1146/annurev.pharmtox.48.113006.094626. PMID 20055686.