The Miller–Urey experiment, or Miller experiment, was an experiment in chemical synthesis carried out in 1952 that simulated the conditions thought at the time to be present in the atmosphere of the early, prebiotic Earth. It is seen as one of the first successful experiments demonstrating the synthesis of organic compounds from inorganic constituents in an origin of life scenario. The experiment used methane (CH4), ammonia (NH3), hydrogen (H2), in ratio 2:2:1, and water (H2O). Applying an electric arc (simulating lightning) resulted in the production of amino acids.
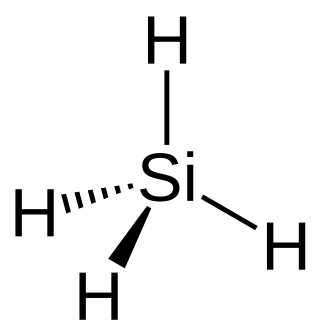
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colorless, pyrophoric, toxic gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter.

Gabor A. Somorjai is a professor of chemistry at the University of California, Berkeley, and is a leading researcher in the field of surface chemistry and catalysis, especially the catalytic effects of metal surfaces on gas-phase reactions. For his contributions to the field, Somorjai won the Wolf Prize in Chemistry in 1998, the Linus Pauling Award in 2000, the National Medal of Science in 2002, the Priestley Medal in 2008, the 2010 BBVA Foundation Frontiers of Knowledge Award in Basic Science and the NAS Award in Chemical Sciences in 2013. In April 2015, Somorjai was awarded the American Chemical Society's William H. Nichols Medal.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.

Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
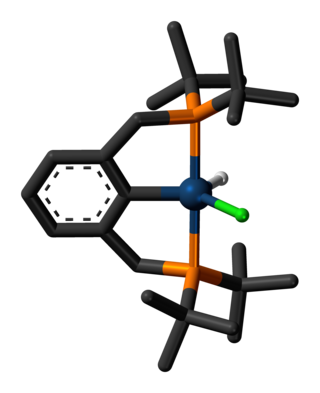
In chemistry, a transition metal pincer complex is a type of coordination complex with a pincer ligand. Pincer ligands are chelating agents that binds tightly to three adjacent coplanar sites in a meridional configuration. The inflexibility of the pincer-metal interaction confers high thermal stability to the resulting complexes. This stability is in part ascribed to the constrained geometry of the pincer, which inhibits cyclometallation of the organic substituents on the donor sites at each end. In the absence of this effect, cyclometallation is often a significant deactivation process for complexes, in particular limiting their ability to effect C-H bond activation. The organic substituents also define a hydrophobic pocket around the reactive coordination site. Stoichiometric and catalytic applications of pincer complexes have been studied at an accelerating pace since the mid-1970s. Most pincer ligands contain phosphines. Reactions of metal-pincer complexes are localized at three sites perpendicular to the plane of the pincer ligand, although in some cases one arm is hemi-labile and an additional coordination site is generated transiently. Early examples of pincer ligands were anionic with a carbanion as the central donor site and flanking phosphine donors; these compounds are referred to as PCP pincers.

Molecular machines are a class of molecules typically described as an assembly of a discrete number of molecular components intended to produce mechanical movements in response to specific stimuli, mimicking macromolecular devices such as switches and motors. Naturally occurring or biological molecular machines are responsible for vital living processes such as DNA replication and ATP synthesis. Kinesins and ribosomes are examples of molecular machines, and they often take the form of multi-protein complexes. For the last several decades, scientists have attempted, with varying degrees of success, to miniaturize machines found in the macroscopic world. The first example of an artificial molecular machine (AMM) was reported in 1994, featuring a rotaxane with a ring and two different possible binding sites. In 2016 the Nobel Prize in Chemistry was awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart, and Bernard L. Feringa for the design and synthesis of molecular machines.

Jack David Dunitz FRS was a British chemist and widely known chemical crystallographer. He was Professor of Chemical Crystallography at the ETH Zurich from 1957 until his official retirement in 1990. He held Visiting Professorships in the United States, Israel, Japan, Canada, Spain and the United Kingdom.

Frank Henry Westheimer was an American chemist. He taught at the University of Chicago from 1936 to 1954, and at Harvard University from 1953 to 1983, becoming the Morris Loeb Professor of Chemistry in 1960, and Professor Emeritus in 1983. The Westheimer medal was established in his honor in 2002.

Arieh Warshel is an Israeli-American biochemist and biophysicist. He is a pioneer in computational studies on functional properties of biological molecules, Distinguished Professor of Chemistry and Biochemistry, and holds the Dana and David Dornsife Chair in Chemistry at the University of Southern California. He received the 2013 Nobel Prize in Chemistry, together with Michael Levitt and Martin Karplus for "the development of multiscale models for complex chemical systems".

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.

Daniel George Nocera is an American chemist, currently the Patterson Rockwood Professor of Energy in the Department of Chemistry and Chemical Biology at Harvard University. He is a member of the National Academy of Sciences and the American Academy of Arts and Sciences. In 2006 he was described as a "major force in the field of inorganic photochemistry and photophysics". Time magazine included him in its 2009 list of the 100 most influential people.
Graham R. Fleming is a professor of chemistry at the University of California, Berkeley and member of the Kavli Energy NanoScience Institute based at UCB.

Jean-Marie Tarascon FRSC is professor of chemistry at the Collège de France in Paris and director of the French Research Network on Electrochemical Energy Storage (RS2E).

Bernard Lucas "Ben" Feringa is a Dutch synthetic organic chemist, specializing in molecular nanotechnology and homogeneous catalysis.
Millard Henry Alexander is an American theoretical chemist. He is Distinguished University Professor at the University of Maryland, with appointments in the Department of Chemistry and Biochemistry and the Institute for Physical Science and Technology. He is the author of over 300 publications and an active researcher in the fields of molecular collision dynamics and theoretical chemistry.

Paul James Chirik is an American chemist known for his work in sustainable chemistry using Earth-abundant metals like iron, cobalt, and nickel to surpass the performance of more exotic elements traditionally used in catalysis. He is the Edwards S. Sanford Professor of Chemistry and chair of the chemistry department at Princeton University.
Xile Hu is a Swiss chemist specialized in catalysis. He is a professor in chemistry at EPFL and leads the Laboratory of Inorganic Synthesis and Catalysis at the School of Basic Sciences.
Metal-ligand cooperativity (MLC) is a mode of reactivity in which a metal and ligand of a complex are both involved in the bond breaking or bond formation of a substrate during the course of a reaction. This ligand is an actor ligand rather than a spectator, and the reaction is generally only deemed to contain MLC if the actor ligand is doing more than leaving to provide an open coordination site. MLC is also referred to as "metal-ligand bifunctional catalysis." Note that MLC is not to be confused with cooperative binding.
Carbon-carbon bond activation refers to the breaking of carbon-carbon bonds in organic molecules. This process is an important tool in organic synthesis, as it allows for the formation of new carbon-carbon bonds and the construction of complex organic molecules. However, C–C bond activation is challenging mainly for the following reasons: (1) C-H bond activation is a competitive process of C-C activation, which is both energetically and kinetically more favorable; (2) the accessibility of the transition metal center to C–C bonds is generally difficult due to its 'hidden' nature; (3) relatively high stability of the C–C bond. As a result, in the early stage, most examples of C-C activation are of stringed ring systems, which makes C-C activation more favorable by increasing the energy of the starting material. However, C-C activation of unstrained C-C bonds has remained challenging until the recent two decades.