Related Research Articles

Mitochondrial DNA is the DNA located in the mitochondria organelles in a eukaryotic cell that converts chemical energy from food into adenosine triphosphate (ATP). Mitochondrial DNA is a small portion of the DNA contained in a eukaryotic cell; most of the DNA is in the cell nucleus, and, in plants and algae, the DNA also is found in plastids, such as chloroplasts.
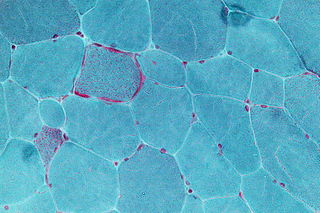
Mitochondrial disease is a group of disorders caused by mitochondrial dysfunction. Mitochondria are the organelles that generate energy for the cell and are found in every cell of the human body except red blood cells. They convert the energy of food molecules into the ATP that powers most cell functions.

Homoplasmy is a term used in genetics to describe a eukaryotic cell whose copies of mitochondrial DNA are all identical. In normal and healthy tissues, all cells are homoplasmic. Homoplasmic mitochondrial DNA copies may be normal or mutated; however, most mutations are heteroplasmic. It has been discovered, though, that homoplasmic mitochondrial DNA mutations may be found in human tumors.

Leber's hereditary optic neuropathy (LHON) is a mitochondrially inherited degeneration of retinal ganglion cells (RGCs) and their axons that leads to an acute or subacute loss of central vision; it predominantly affects young adult males. LHON is transmitted only through the mother, as it is primarily due to mutations in the mitochondrial genome, and only the egg contributes mitochondria to the embryo. Men cannot pass on the disease to their offspring. LHON is usually due to one of three pathogenic mitochondrial DNA (mtDNA) point mutations. These mutations are at nucleotide positions 11778 G to A, 3460 G to A and 14484 T to C, respectively in the ND4, ND1 and ND6 subunit genes of complex I of the oxidative phosphorylation chain in mitochondria.

The Wellcome Sanger Institute, previously known as The Sanger Centre and Wellcome Trust Sanger Institute, is a non-profit British genomics and genetics research institute, primarily funded by the Wellcome Trust.

The Cambridge Reference Sequence (CRS) for human mitochondrial DNA was first announced in 1981.

MT-ND6 is a gene of the mitochondrial genome coding for the NADH-ubiquinone oxidoreductase chain 6 protein (ND6). The ND6 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variations in the human MT-ND6 gene are associated with Leigh's syndrome, Leber's hereditary optic neuropathy (LHON) and dystonia.

MT-ND4 is a gene of the mitochondrial genome coding for the NADH-ubiquinone oxidoreductase chain 4 (ND4) protein. The ND4 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variations in the MT-ND4 gene are associated with age-related macular degeneration (AMD), Leber's hereditary optic neuropathy (LHON), mesial temporal lobe epilepsy (MTLE) and cystic fibrosis.

MT-ND2 is a gene of the mitochondrial genome coding for the NADH dehydrogenase 2 (ND2) protein. The ND2 protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variants of human MT-ND2 are associated with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS), Leigh's syndrome (LS), Leber's hereditary optic neuropathy (LHON) and increases in adult BMI.

MT-ND4L is a gene of the mitochondrial genome coding for the NADH-ubiquinone oxidoreductase chain 4L (ND4L) protein. The ND4L protein is a subunit of NADH dehydrogenase (ubiquinone), which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain. Variants of human MT-ND4L are associated with increased BMI in adults and Leber's Hereditary Optic Neuropathy (LHON).

MT-ATP8 is a mitochondrial gene with the full name 'mitochondrially encoded ATP synthase membrane subunit 8' that encodes a subunit of mitochondrial ATP synthase, ATP synthase Fo subunit 8. This subunit belongs to the Fo complex of the large, transmembrane F-type ATP synthase. This enzyme, which is also known as complex V, is responsible for the final step of oxidative phosphorylation in the electron transport chain. Specifically, one segment of ATP synthase allows positively charged ions, called protons, to flow across a specialized membrane inside mitochondria. Another segment of the enzyme uses the energy created by this proton flow to convert a molecule called adenosine diphosphate (ADP) to ATP. Subunit 8 differs in sequence between Metazoa, plants and Fungi.
Mitochondrially encoded tRNA leucine 1 (UUA/G) also known as MT-TL1 is a transfer RNA which in humans is encoded by the mitochondrial MT-TL1 gene.

DNA polymerase subunit gamma is an enzyme that in humans is encoded by the POLG gene. Mitochondrial DNA polymerase is heterotrimeric, consisting of a homodimer of accessory subunits plus a catalytic subunit. The protein encoded by this gene is the catalytic subunit of mitochondrial DNA polymerase. Defects in this gene are a cause of progressive external ophthalmoplegia with mitochondrial DNA deletions 1 (PEOA1), sensory ataxic neuropathy dysarthria and ophthalmoparesis (SANDO), Alpers-Huttenlocher syndrome (AHS), and mitochondrial neurogastrointestinal encephalopathy syndrome (MNGIE).

Cytochrome c oxidase II is a protein in eukaryotes that is encoded by the MT-CO2 gene. Cytochrome c oxidase subunit II, abbreviated COXII, COX2, COII, or MT-CO2, is the second subunit of cytochrome c oxidase. It is also one of the three mitochondrial DNA (mtDNA) encoded subunits of respiratory complex IV.

Cytochrome c oxidase subunit III (COX3) is an enzyme that in humans is encoded by the MT-CO3 gene. It is one of main transmembrane subunits of cytochrome c oxidase. It is also one of the three mitochondrial DNA (mtDNA) encoded subunits of respiratory complex IV. Variants of it have been associated with isolated myopathy, severe encephalomyopathy, Leber hereditary optic neuropathy, mitochondrial complex IV deficiency, and recurrent myoglobinuria.

Twinkle protein also known as twinkle mtDNA helicase is a mitochondrial protein that in humans is encoded by the TWNK gene located in the long arm of chromosome 10 (10q24.31).
Mitochondrially encoded tRNA valine also known as MT-TV is a transfer RNA which in humans is encoded by the mitochondrial MT-TV gene.
Mitochondrially encoded tRNA phenylalanine also known as MT-TF is a transfer RNA which in humans is encoded by the mitochondrial MT-TF gene.
Mitochondrial replacement therapy (MRT), sometimes called mitochondrial donation, is the replacement of mitochondria in one or more cells to prevent or ameliorate disease. MRT originated as a special form of in vitro fertilisation in which some or all of the future baby's mitochondrial DNA (mtDNA) comes from a third party. This technique is used in cases when mothers carry genes for mitochondrial diseases. The therapy is approved for use in the United Kingdom. A second application is to use autologous mitochondria to replace mitochondria in damaged tissue to restore the tissue to a functional state. This has been used in clinical research in the United States to treat cardiac-compromised newborns.
Patrick Francis Chinnery is a neurologist, clinician scientist, and Wellcome Trust Principal Research Fellow based in the Medical Research Council Mitochondrial Biology Unit and the University of Cambridge, where he is also professor of neurology and head of the department of clinical neurosciences.
References
- ↑ "Turnbull, Professor Douglass M". newcastle-hospitals.org.uk. Archived from the original on 2015-06-25.
- 1 2 3 4 "Professor Doug Turnbull: Personal Biography". newcastle-mitochondria.com. Newcastle upon Tyne. 17 March 2016. Archived from the original on 2016-03-31.
- 1 2 Craven, Lyndsey; Tuppen, Helen A.; Greggains, Gareth D.; Harbottle, Stephen J.; Murphy, Julie L.; Cree, Lynsey M.; Murdoch, Alison P.; Chinnery, Patrick F.; Taylor, Robert W.; Lightowlers, Robert N.; Herbert, Mary; Turnbull, Douglass M. (2010). "Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease". Nature. 465 (7294): 82–85. Bibcode:2010Natur.465...82C. doi:10.1038/nature08958. PMC 2875160 . PMID 20393463.

- ↑ Healing broken batteries: The Wellcome Trust Centre for Mitochondrial Research on YouTube, Wellcome Trust, London
- ↑ Graeme Whitfield (2015). "Newcastle University medical pioneer Doug Turnbull discusses his game-changing research". thejournal.co.uk. Newcastle: The Journal. Archived from the original on 2020-08-18. Retrieved 2016-06-16.
- ↑ Turnbull, Douglass Matthew (1983). Mitochondrial cytopathies: clinical and experimental studies (PhD thesis). Newcastle upon Tyne University. OCLC 11274373.
- ↑ Lightowlers, Robert N.; Chinnery, Patrick F.; Turnbull, Douglass M.; Howell, Neil (1997). "Mammalian mitochondrial genetics: heredity, heteroplasmy and disease". Trends in Genetics. 13 (11): 450–455. doi:10.1016/S0168-9525(97)01266-3. PMID 9385842.
- ↑ Douglass Turnbull's publications indexed by the Scopus bibliographic database. (subscription required)
- ↑ Turnbull, Douglass M.; Andrews, Richard M.; Kubacka, Iwona; Chinnery, Patrick F.; Lightowlers, Robert N.; Howell, Neil (1999). "Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA". Nature Genetics. 23 (2): 147. doi: 10.1038/13779 . PMID 10508508.
- ↑ Bender, Andreas; Krishnan, Kim J; Morris, Christopher M; Taylor, Geoffrey A; Reeve, Amy K; Perry, Robert H; Jaros, Evelyn; Hersheson, Joshua S; Betts, Joanne; Klopstock, Thomas; Taylor, Robert W; Turnbull, Douglass M (2006). "High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease". Nature Genetics. 38 (5): 515–517. doi:10.1038/ng1769. PMID 16604074. S2CID 13956928.
- ↑ Taylor, Robert W.; Turnbull, Doug M. (2005). "Mitochondrial DNA mutations in human disease". Nature Reviews Genetics. 6 (5): 389–402. doi:10.1038/nrg1606. PMC 1762815 . PMID 15861210.
- ↑ Herrnstadt, Corinna; Elson, Joanna L.; Fahy, Eoin; Preston, Gwen; Turnbull, Douglass M.; Anderson, Christen; Ghosh, Soumitra S.; Olefsky, Jerrold M.; Beal, M. Flint; Davis, Robert E.; Howell, Neil (2002). "Reduced-Median-Network Analysis of Complete Mitochondrial DNA Coding-Region Sequences for the Major African, Asian, and European Haplogroups". The American Journal of Human Genetics. 70 (5): 1152–1171. doi:10.1086/339933. PMC 447592 . PMID 11938495.

- ↑ Taylor, Robert W.; Barron, Martin J.; Borthwick, Gillian M.; Gospel, Amy; Chinnery, Patrick F.; Samuels, David C.; Taylor, Geoffrey A.; Plusa, Stefan M.; Needham, Stephanie J.; Greaves, Laura C.; Kirkwood, Thomas B.L.; Turnbull, Douglass M. (2003). "Mitochondrial DNA mutations in human colonic crypt stem cells". Journal of Clinical Investigation. 112 (9): 1351–1360. doi:10.1172/JCI19435. PMC 228466 . PMID 14597761.

- ↑ "UK Government grants awarded to Doug Turnbull". rcuk.ac.uk. Swindon: Research Councils UK. Archived from the original on 2016-06-16.
- ↑ "Professor Doug Turnbull FMedSci". acmedsci.ac.uk. London: Academy of Medical Sciences. Archived from the original on 2016-06-16.
- ↑ "No. 61608". The London Gazette (Supplement). 2016-06-11. p. B2.
- ↑ Anon (2016). "Birthday honours: Mitochondrial disease doctor recognised". bbc.co.uk. London: BBC News.
- ↑ Mark Henderson (2015). "Three-person embryos: how the mitochondrial donation battle was won. Prof Doug Turnbull successfully communicated difficult and controversial research with scientific accuracy, but in simple terms". The Guardian . London. Archived from the original on 2016-02-05.
- ↑ James Gallagher (2015). "Three-person babies - not three-parent babies". bbc.co.uk. London: BBC News. Archived from the original on 2016-03-02.
- ↑ "Buchanan Medallist 2020". Royal Society. Archived from the original on 23 October 2019. Retrieved 18 August 2020.