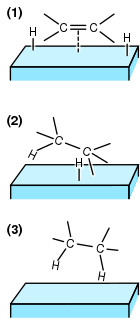
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
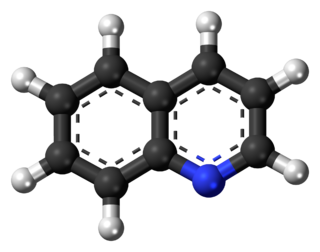
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:

BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1′ positions. This C2-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation (atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle between the naphthyl groups is approximately 90°. The natural bite angle is 93°.

Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes and is named after its inventor Herbert Lindlar.

The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which an achiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with Ammonia in the presence of potassium cyanide. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used commercially for the production of racemic methionine from methional.

Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances.
The Friedländer synthesis is a chemical reaction of 2-aminobenzaldehydes with ketones to form quinoline derivatives. It is named after German chemist Paul Friedländer (1857–1923).
Transfer hydrogenation is the addition of hydrogen (H2; dihydrogen in inorganic and organometallic chemistry) to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2. One large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin.

In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds. Because of their similarity in composition and description, they are often mistaken as a misnomer for enzymes due to their comparable effects on reaction rates and forms of catalysis involved.
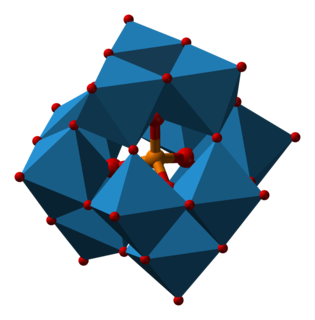
Phosphomolybdic acid is the heteropolymetalate with the formula H3Mo12PO40·12H2O. It is a yellow solid, although even slightly impure samples have a greenish coloration. It is also known as dodeca molybdophosphoric acid or PMA, is a yellow-green chemical compound that is freely soluble in water and polar organic solvents such as ethanol. It is used as a stain in histology and in organic synthesis.

Quinaldine or 2-methylquinoline is an organic compound with the formula CH3C9H6N. It is one of the methyl derivatives of the heterocyclic compound quinoline. It is bioactive and is used in the preparation of various dyes. It is a colorless oil but commercial samples can appear colored.

1,2-Diphenyl-1,2-ethylenediamine, DPEN, is an organic compound with the formula H2NCHPhCHPhNH2, where Ph is phenyl (C6H5). DPEN exists as three stereoisomers: meso and two enantiomers S,S- and R,R-. The chiral diastereomers are used in asymmetric hydrogenation. Both diastereomers are bidentate ligands.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being awarded one half of the 2001 Nobel Prize in Chemistry.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective applications.

3-Hydroxytetrahydrofuran is a colorless liquid with a normal boiling point of 179 °C and boiling at 88−89 °C at 17 mmHg, with density. 3-OH THF is a useful pharmaceutical intermediate. The chiral version of this compound is an intermediate to launched retroviral drugs.
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal organometallics, the chemistry of organoniobium compounds most closely resembles that of organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, -1, and -3 have been prepared, with the +5 oxidation state being the most common.















